After failing to show non-inferiority to ranibizumab at 8 weeks late last year, ONS-5010’s new 12-week data strengthens the case for its upcoming BLA.
Outlook Therapeutics (New Jersey, United States) has announced the completion of the 12-week safety and efficacy analysis for the NORSE EIGHT clinical trial, the second of two studies evaluating ONS-5010 (bevacizumab gamma) for the treatment of wet age-related macular degeneration (AMD).
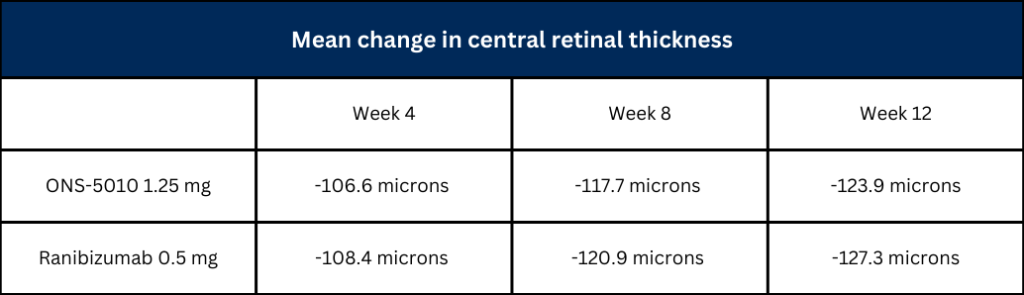
According to a news release from the company, the 12-week data has shown non-inferiority in ONS-5010 was non-inferior to ranibizumab at week 12, with changes in central retinal thickness, a measure of anatomical response, comparable in both study arms across all three time points.1
In November 2024, the company announced that ONS-5010 did not demonstrate non-inferior BCVA gains compared to ranibizumab at week 8 in the Phase III NORSE EIGHT trial. However, best-corrected visual acuity (BCVA) data across all study time points showed consistent improvement in vision over time and indicated the presence of biologic activity.
Dr. Julia A. Haller, ophthalmologist-in-chief at Wills Eye Hospital (Philadelphia, Pennsylvania, USA) and member of Outlook Therapeutics’ board of directors, commented on the data.
“The 3-month data from NORSE EIGHT provides additional evidence to confirm what retina specialists expected. The clinical trial continues to demonstrate that ONS-5010 injections result in immediate and sustained anatomic efficacy, with steady gains in visual acuity and reliable, consistent safety.”
On the heels of the 12-week data, the company has restated its plans to submit a Biologics License Application (BLA) for ONS-5010 in the first quarter of 2025.
“We believe that the statistically significant 12-week results for ONS-5010 in NORSE EIGHT, combined with the complete NORSE EIGHT data set, confirms our successful NORSE TWO pivotal study and will support the resubmission of our BLA in the United States for the treatment of wet AMD,” said Lawrence Kenyon, chief financial officer and interim chief executive officer of Outlook Therapeutics.
Breaking down the data
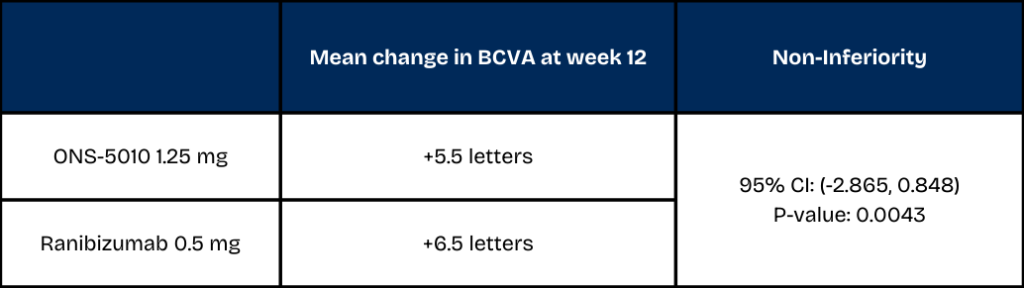
In the NORSE EIGHT trial, ONS-5010 achieved mean visual acuity improvements of +3.3 letters at week 4, +4.2 letters at week 8 and +5.5 letters at week 12. The mean improvement in the ranibizumab arm was +6.5 letters at week 12. The difference in best-corrected visual acuity (BCVA) between ONS-5010 and ranibizumab was -1.009 letters, with a 95% confidence interval of (-2.865, 0.848).
Based on the statistical parameters set forth in the special protocol assessment (SPA) with the U.S. Food and Drug Administration (FDA), the trial met the non-inferiority margin at week 12 (p=0.0043).
The change in central retinal thickness, an indicator of anatomical response, was comparable in both study arms across all three time points.
ONS-5010 demonstrated a reasonable safety profile throughout the NORSE EIGHT trial, with comparable ocular adverse event rates to ranibizumab. No cases of retinal vasculitis were reported in either study arm. The safety results observed in NORSE EIGHT were consistent with previously reported outcomes from the NORSE ONE, NORSE TWO and NORSE THREE trials.2
What’s next for ONS-5010?
The therapy has already received regulatory approval in the European Union and the United Kingdom as LYTENAVA (bevacizumab gamma) and is slated for launch in Europe in the first half of 2025.
On the same day, the company also announced that it has entered into warrant inducement transactions anticipated to raise $20.4 million in gross proceeds to strengthen its cash position ahead of its launch in Europe and prospective FDA approval.
With these results, Outlook Therapeutics is positioning ONS-5010 as a potential replacement for off-label repackaged bevacizumab, which hasn’t been approved for retinal diseases in the U.S.
Editor’s Note: This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
References
1. Outlook Therapeutics Press Release. Available at: https://ir.outlooktherapeutics.com/news-releases/news-release-details/outlook-therapeuticsr-announces-complete-twelve-week-efficacy Accessed on January 17, 2025
2. ONS-5010 Clinical Progress. Available at: https://outlooktherapeutics.com/lytenava-clinical-progress/ Accessed on January 17, 2025