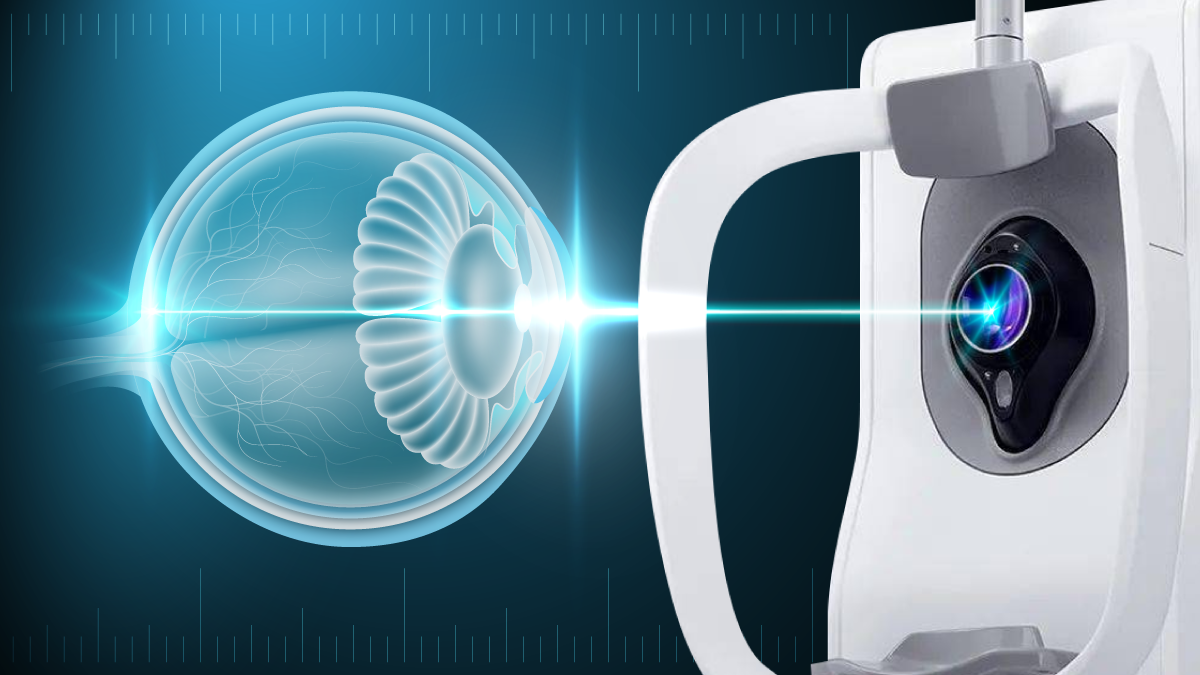
DREAM OCT scans from cornea to vitreous in one single scan, enabling full anterior segment imaging.
Intalight (Shanghai, China), a developer of advanced ophthalmic imaging technologies, has received CE mark approval for its DREAM OCT platform, a next-generation swept-source optical coherence tomography (SS-OCT) system. The certification allows the company to commercialize its technology across the European Union.
“Intalight is incredibly proud to receive CE mark approval in Europe for our DREAM OCT,” said Shawn Peng, the company’s chairman and founder. “This achievement allows us to provide ophthalmologists in Europe with state-of-the-art technology that delivers improved results for their patients.”
READ MORE: Seeing the Unseen: Adaptive Optics Enters the Clinical Arena at ARVO 2025
Features and imaging capabilities
The DREAM OCT platform—an acronym for Deep imaging depth, Rapid sweeping speed, Extensive scan range, Accurate results and Multimodal imaging—is designed to provide highly detailed visualization of the retina, choroid and anterior segment. It offers ultra-widefield imaging, including a 130° OCTA scan and super-depth 12 mm scanning, enabling clinicians to image from the cornea to the anterior vitreous in a single pass.
According to Intalight, the platform also improves penetration through opacities in the lens and vitreous thanks to its longer wavelength light source, a feature aimed at delivering clearer images even in difficult cases.
READ MORE: Adding Functional Testing to Structural Imaging in Retinal Disease Management
Designed for clinical use in challenging cases
“Over the past few years, we’ve heard from eye care professionals that they need a solution that gets them over the imaging finish line with speed, accuracy and depth,” said Bing Li, Intalight’s CEO and co-founder. “DREAM OCT delivers a full set of imaging modalities for the most challenging clinical and research applications for the retina and outperforms everything else HCPs know.”
DREAM OCT capturing a rare congenital benign tumor with high-definition clarity
Already used in over 160 peer-reviewed studies, DREAM OCT has been adopted by academic and private retina practices across the United States, Asia and Europe. The CE mark represents a key step for Intalight as it looks to expand its footprint in global ophthalmology.
Next regulatory steps
“We look forward to continuing to grow our prestigious network of global institutions and ophthalmologists with our recent CE approval and eventually our FDA approval,” said Joe Garibaldi, chief commercial officer.
With EU clearance secured, Intalight’s focus now turns to regulatory pathways in other key markets, including the United States.
Editor’s note:
- For more information, see Topcon Intalight’s press release on the DREAM OCT.
- This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.