On Day 3 of the 25th Congress of the European Society of Retina Specialists (EURETINA 2025) in Paris, the Grand Amphitheater lit up with innovation as the Current Status of Innovative Therapies for Inherited Retinal Disorders session unfolded under the guidance of Prof. Dr. Isabelle Audo (France) and Dr. Bart LeRoy (Belgium).
Eight speakers charted a course through gene replacement and editing, CRISPR and antisense oligonucleotides, cell therapy, optogenetics and gene-agnostic pipelines. The discussion mixed technical tenacity with clinical imagination, balancing delivery routes, trial design and patient selection. The result was a Parisian tableau of progress, where IRDs look not only inheritable, but increasingly interruptible.
READ MORE: The Promise of Gene Therapy in Retinal Disorders
Gene therapy for RPE65-IRD in practice
Prof. Dominik Fischer (UK) opened the session with pragmatic advice on delivering gene therapy for RPE65-mediated IRDs. Real-world data, including the PERCEIVE study, show only modest gains in visual acuity overall, though younger patients often see greater benefit, especially in light sensitivity.
“From the PERCEIVE study, the real-world evidence suggests that there is not going to be a significant change of your best-corrected visual acuity overall,” he noted. “However, that does depend on the age and, of course, the disease stage in which you start treating.”
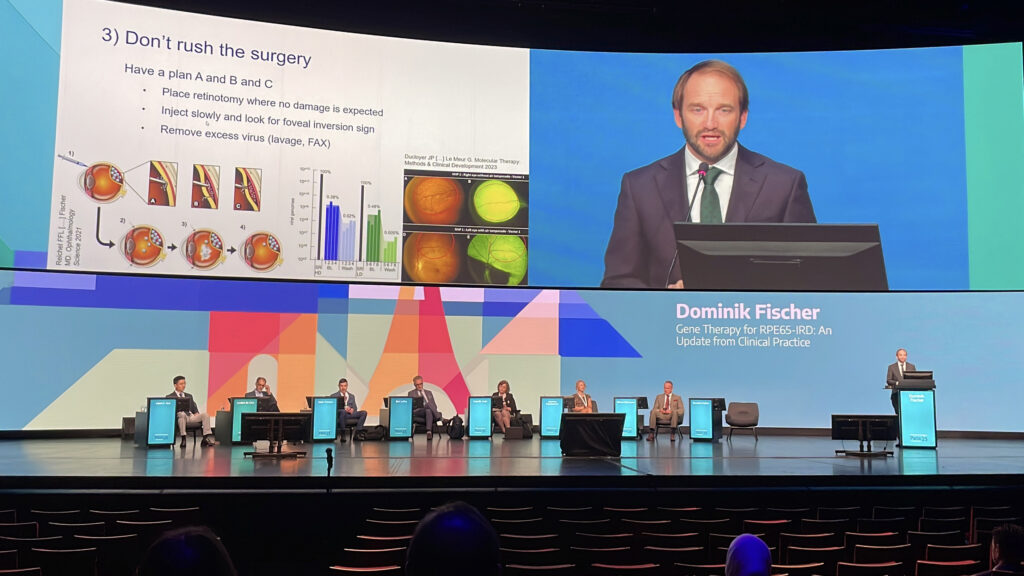
Surgical nuance mattered, like slow subretinal injection, careful cannula placement and a virus lavage help reduce spillover and inflammation. Still, side effects like gene therapy-associated uveitis, atrophy and steroid-induced ocular hypertension still call for close monitoring. Prof. Fischer’s takeaway was to choose patients wisely, set realistic expectations and never skip the fluid-air exchange.
READ MORE: One Day, Three IRD Gene Therapy Triumphs
Rewinding degeneration in X-linked RP
Prof. Dr. Robert MacLaren (UK) presented compelling results from RPGR gene therapy in X-linked retinitis pigmentosa (XLRP), a condition he described as one of the most common and severe IRDs.
Subretinal delivery of a codon-optimized full-length RPGR transgene produced clear anatomical recovery, including reappearance of the external limiting membrane and thickening of photoreceptor layers, together with functional gains in retinal sensitivity. In some patients, improvement was visible within three months of treatment.
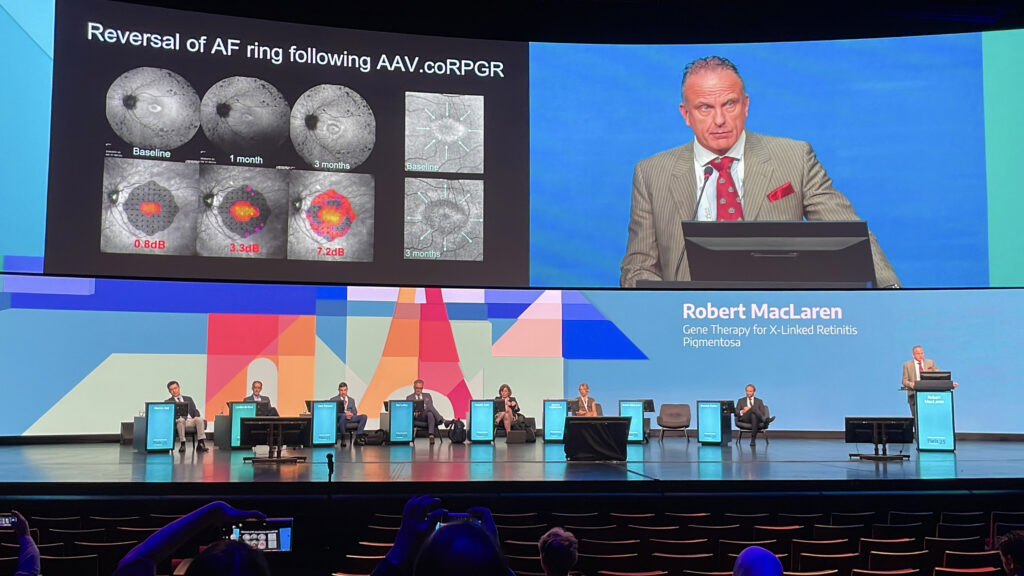
“The anatomical reversal we’ve seen in RPGR gene therapy is an incredibly important sign,” Prof. Dr. MacLaren noted, “and much more objective than functional tests.” Importantly, no evidence suggested that proximal RPGR mutations impaired outcomes. His conclusion was that, for the first time, reversal of retinal degeneration in XLRP appears not only possible but measurable.
READ MORE: First Patients Dosed in jCyte’s Phase II JC02-88 Trial for Retinitis Pigmentosa
Lessons from choroideremia trials
Dr. Jasmina Kapetanovic (UK) offered a frank look at gene therapy for choroideremia, an X-linked degeneration caused by REP1 deficiency. In the multicenter STELLAR and REGENERATE trials, treatment proved safe but failed to deliver statistically significant gains in visual acuity, though trends favored earlier intervention. Patients with preserved autofluorescence and central retinal structure responded best, and microperimetry proved more sensitive than BCVA as an endpoint.

“What I would like to draw attention to here is how thin and fragile that retina is in choroideremia,” Dr. Kapetanovic reminded the audience. “And it’s up to us surgeons to detach this retina safely without further iatrogenic damage.”
She also pointed to advances on the horizon, including optogenetic therapies and robotic subretinal delivery, both of which may help overcome current biological and surgical obstacles.
Outsmarting size limits in IRD gene therapy
Dr. Bart LeRoy (Belgium) tackled one of the toughest barriers in inherited retinal disease (IRD) gene therapy: what to do when the target gene is simply too big for an adeno-associated virus (AAV) vector. For oversized genes like CEP290, antisense oligonucleotides (AONs) provide a vector-free workaround by correcting splicing errors with short RNA molecules, delivered intravitreally and dosed repeatedly.
“The capacity of AAV vectors is limited,” Dr. LeRoy explained. “It’s like a little delivery can[ister] that can only deliver so much. So there needs to be technology that is able to be used for large genes.”

Other approaches on the table include dual-vector approaches that split genes such as MYO7A and ABCA4, along with RNA trans-splicing, which repairs faulty mRNA directly. Together, these strategies are pushing the therapeutic horizon for patients previously considered untreatable.
READ MORE: Gene Therapy Shows Promise for AIPL1-Associated Retinal Dystrophy in Children
Gene editing takes aim at CEP290-IRD
Dr. Mark Pennesi (USA) presented long-term results from the BRILLIANCE trial, the first in vivo CRISPR/Cas9 therapy tested in humans, targeting CEP290-related Leber congenital amaurosis (LCA). Patients with this severe retinal dystrophy often retain structure despite profound vision loss, making them prime candidates for editing. EDIT-101, delivered subretinally via AAV5, removes the intronic mutation that disrupts protein function.
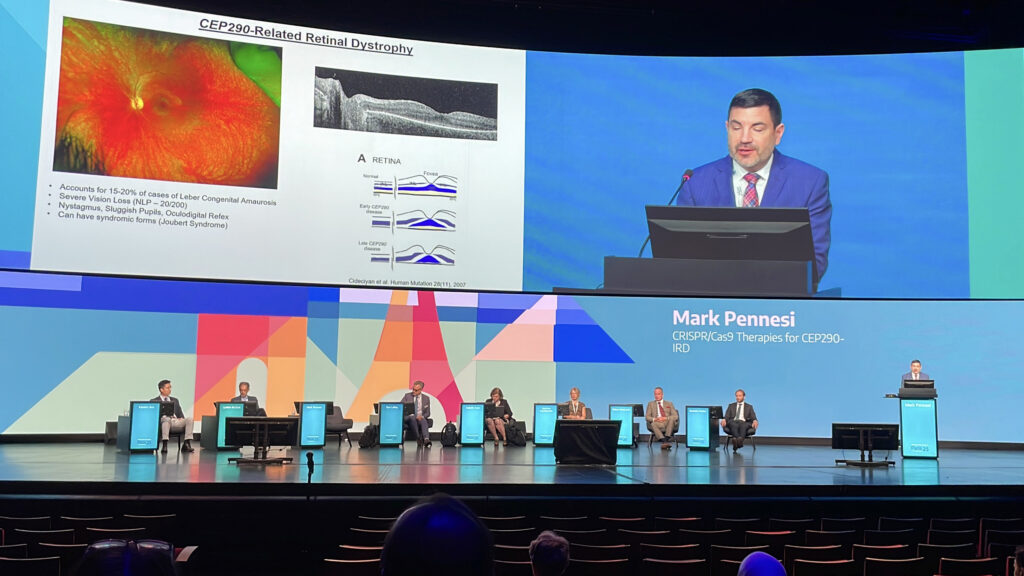
At one year, four of 14 patients gained ≥ 0.3 logMAR in visual acuity, six improved on full-field sensitivity testing and four performed better in mobility navigation. “We do see functional improvement with EDIT-101,” Dr. Pennesi told the audience, “with nearly 80% of patients showing gains in at least one metric.” Benefits persisted up to four years in most responders, bringing home the durable potential of gene editing in IRDs.
Agnostic approaches to IRD treatment
Prof. Dr. Isabelle Audo (France) featured gene-agnostic therapies as a way to tackle the vast heterogeneity of IRDs. Instead of correcting one mutation, these strategies target universal processes to preserve or restore vision.
“The rationale for gene-agnostic therapy is the clinical, but also, and more importantly, the genetic heterogeneity of IRDs,” she explained. “The idea is then not to correct a mutation or a gene, but to act through universal phenomenon.”

For neuroprotection, she pointed to NXNL1-based therapies such as SPPN06 in the ProDigi trial, which enhance glucose metabolism and counter oxidative stress. AAV-delivered NR2E3 (Ocugen) has also shown early signals of efficacy.
In advanced degeneration, optogenetics hope to repurpose ganglion cells with channelrhodopsin, while GRK1 transfer (SPPN20) seeks to revive dormant cones. Though early, these therapies promise new options for patients beyond gene-specific solutions.
READ MORE: FDA Clears Ocugen’s Phase II/III Trial for Stargardt Disease Gene Therapy
Rebuilding vision from the ground up
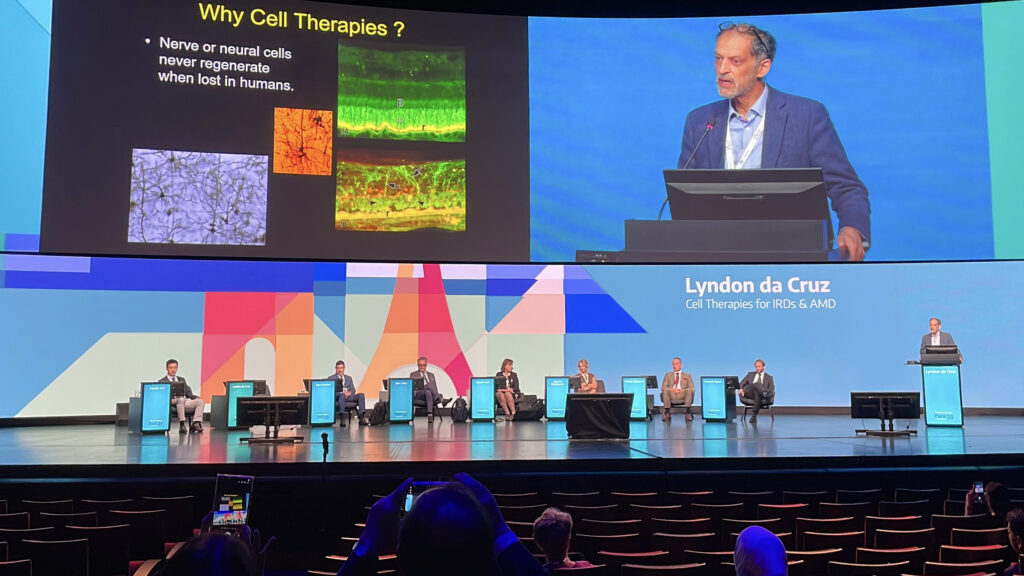
Prof. Lyndon da Cruz (UK) outlined progress in cell-based therapies for IRDs and age-related macular degeneration (AMD). The concept is that when retinal cells are lost, replacing them may restore function.
Researchers are exploring sources including embryonic stem cells, induced pluripotent stem cells (iPSCs) and adult progenitors to engineer retinal pigment epithelium (RPE) sheets, suspensions and even retinal organoids.
Delivery is still a surgical challenge, with trials testing subretinal injection, patch implantation and robotic assistance. “Since 2012, more than 100 patients have received some form of published stem cell transplantation,” Prof. da Cruz reported. “There’s no major safety signal in terms of tumor and immune reaction.”
Early results suggest persistence and safety, though efficacy remains limited. Even so, as techniques mature, cell therapy is inching closer to clinical reality.
Inflammation at the frontier
Dr. Kanmin Xue (UK) closed the session with a reminder that as gene therapy moves from rare IRDs into common conditions like AMD and diabetic retinopathy (DR), inflammation management must move to the forefront. Gene therapy-associated uveitis (GTAU) varies by delivery route, with intravitreal injections often triggering anterior chamber reactions and subretinal approaches producing localized infiltrates.
“Gene therapy is becoming more common,” Dr. Xue said, “and initially we started offering rare diseases such as IRDs, but now several clinical trials are leading to advanced stages for developing similar treatments for more common diseases such as AMD and DR. Therefore, controlling inflammation has become much more relevant clinically.”

Strategies under study include improved vector design, personalized immunosuppression and suprachoroidal delivery. Proactive monitoring, tailored steroid regimens and trial endpoints that balance safety with efficacy will be key as the field scales up.
READ MORE: AI System Eye2Gene Shows Promise in Rapid Genetic Diagnosis of IRDs
A future in sight
This session unveiled a field in full stride, powered by deeper genetic insight, smarter delivery systems and growing clinical experience. Gene replacement is entering real-world practice, while editing, agnostic and optogenetic strategies push into new territory. Cell-based approaches are edging closer to viability and surgical finesse is steadily improving.
Challenges like inflammation, delivery limits and trial design linger, but the collective momentum is undeniable. Therapies are maturing, patients are being enrolled earlier and conversations with regulators are happening. For conditions once thought irreversible, the pipeline now carries more than proof of concept. It carries proof of possibility.
Editor’s Note: The 25th EURETINA Congress was held from 4-7 September, in Paris, France. Reporting for this story took place during the event. This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
