A new retinal dystrophy gene therapy brings hope—restoring sight to blind kids and rewriting futures.
A study published in The Lancet on Feb 22, 2025, has demonstrated the potential of gene therapy to improve vision in children with AIPL1-associated severe retinal dystrophy, a rare and rapidly progressing condition causing blindness from birth.1
The first-in-human clinical trial, conducted in the United Kingdom, involved four children aged 1.0 to 2.8 years with biallelic AIPL1 gene mutations.
Researchers administered a recombinant adeno-associated viral vector (rAAV8.hRKp.AIPL1) via subretinal injection to one eye of each child. The treatment was designed to provide a functional copy of the AIPL1 gene to affected retinal cells.1
AIPL-1 treatment findings
At a mean follow-up of 3.5 years post-treatment, all four children showed significant improvements in visual acuity in the treated eyes.
Before intervention, their vision was limited to light perception. Following treatment, visual acuity in the treated eyes improved to a mean of 0.9 logMAR (ranging from 0.8 to 1.0), whereas vision in the untreated eyes deteriorated further, becoming unmeasurable at the final assessment. Two children who were able to undergo objective testing confirmed improved visual function, and electrophysiological recordings showed enhanced activity in the visual cortex of the treated eyes.1
In addition to functional gains, retinal imaging revealed better structural preservation in the treated eyes. Three children demonstrated improved outer retinal lamination, and retinal thickness appeared more stable compared to untreated eyes.
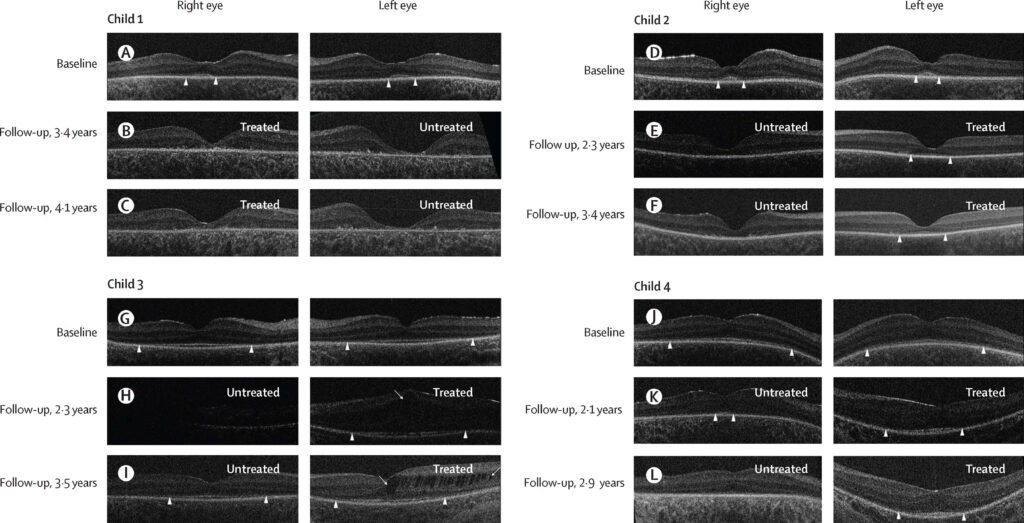
One child developed cystoid macular edema in the treated eye, which partially resolved by the last follow-up, but no other significant safety concerns were observed.1
Implications for retinal dystrophy
The study, funded by the UK National Institute for Health Research and Moorfields Eye Charity, provides the first clinical evidence that early gene therapy intervention may restore vision and slow retinal degeneration in children with AIPL1-associated retinal dystrophy.
Researchers suggest that earlier intervention could yield even greater benefits by preserving retinal architecture and visual function during critical developmental years.1
While the results are promising, the study authors acknowledge the limitations of a small sample size and the absence of a control group. However, they note that the magnitude of improvement and objective measures of retinal structure and function support the therapy’s potential efficacy.1
The findings highlight gene therapy’s potential for treating other genetic retinal diseases, particularly when administered at an early stage. The world now awaits further research and larger clinical trials to confirm the long-term benefits and broader applicability of this approach to pediatric patients with other inherited retinal disorders.
Editor’s Note: This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
Reference
- Michaelides M, Laich Y, Wong SC, et al. Gene therapy in children with AIPL1-associated severe retinal dystrophy: An open-label, first-in-human interventional study. Lancet. 2025;405(10479):648-657.



