Regeneron’s proposal to lengthen EYLEA HD dosing intervals to 24 weeks hits a CRL roadblock.
The wait for extended treatment intervals with anti-VEGF agents in exudative retinal diseases continues.
Regeneron (New Jersey, United States) announced Friday that the U.S. Food and Drug Administration (FDA) has issued a Complete Response Letter (CRL) in response to the company’s supplemental Biologics License Application (sBLA) for EYLEA HD (aflibercept 8 mg).
The CRL essentially ends the firm’s current efforts (for now) to extend dosing intervals up to every 24 weeks across all approved indications, including diabetic macular edema (DME) and neovascular age-related macular degeneration (nAMD).
READ MORE: Bayer’s Aflibercept 8 mg Eyes Six-Month Stretch Between Treatments
While the firm’s press release noted that the FDA raised no new safety or efficacy concerns with the EYLEA HD’s current labelling, the agency concluded that the evidence presented did not support the proposed label update.
The move keeps Regeneron’s higher-dose anti-VEGF therapy on its current schedule, pending further clinical data.
Takeaways for eyecare professionals
- The FDA declined Regeneron’s bid to extend EYLEA HD intervals to 24 weeks
- No new safety or efficacy issues were identified
- Trial data from PULSAR and PHOTON showed non-inferiority at 12- and 16-week intervals
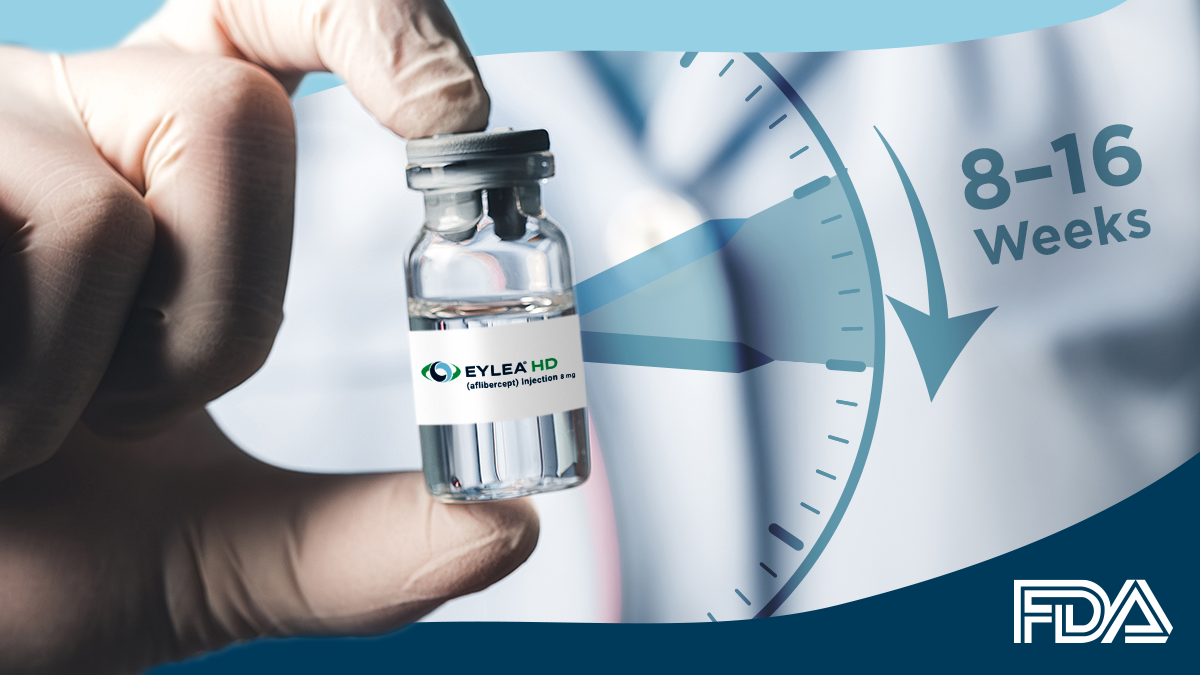
- Current dosing guidance (8–16 weeks) remains unchanged
PULSAR and PHOTON: Promising, but not persuasive enough
Regeneron’s application was supported by data from two Phase 3 studies: PULSAR, focused on neovascular age-related macular degeneration (nAMD), and PHOTON, evaluating patients with diabetic macular edema (DME).1,2
Both trials tested the efficacy of EYLEA HD dosed every 12 or 16 weeks, compared with standard aflibercept 2 mg dosed every 8 weeks. At 48 weeks, both studies demonstrated non-inferior gains in best-corrected visual acuity (BCVA), establishing the potential for lower treatment burden without sacrificing vision outcomes.
In PULSAR, 77% of patients in the 16-week arm maintained their interval through Week 48. In PHOTON, that number was even higher: 89% maintained their extended dosing schedule. Across both trials, these patients also showed stable anatomical outcomes on OCT imaging and a comparable safety profile.
READ MORE: Bayer’s Eylea 8mg Makes Huge Regulatory Strides Worldwide
Regeneron argued that these findings provided compelling support for a broader label that would allow clinicians to tailor treatment schedules up to 24 weeks in appropriate patients.
But the FDA’s position was clear: although promising, the available evidence wasn’t sufficient to justify a 24-week interval across indications. The agency did not call into question the integrity of the data but stated that further evidence would be needed to support such an expansion.
What this means for clinical practice
At present, EYLEA HD remains approved on the following schedule:
- For wAMD and DME: 8 to 16 weeks after an initial series of three monthly injections
- For diabetic retinopathy (DR): 8 to 12 weeks following loading doses
This dosing flexibility is still broader than that of standard-dose aflibercept and provides retinal specialists with some latitude in reducing injection burden. However, the FDA’s response highlights the level of scrutiny required when proposing changes that could shift treatment behavior on a wide scale.
The FDA’s decision is likely to be viewed by many clinicians as a sign that the agency is holding the line on evidence thresholds for extended interval dosing. Although the data from PULSAR and PHOTON were encouraging, the CRL suggests regulators are looking for longer follow-up and greater reproducibility before greenlighting changes that could reshape treatment patterns.3
Retinal specialists are likely to interpret the FDA’s rejection as a signal that extended dosing intervals must be supported by even more long-term data before being integrated into standard practice. While the 24-week interval was appealing from a burden-reduction standpoint, the agency’s response suggests that sustained outcomes and reproducibility across broader populations will remain essential to securing future label changes.
Many clinicians had hoped that approval of the 24-week interval would allow for even greater personalization of treatment schedules, especially for patients demonstrating long-term stability. Some viewed the 24-week mark as a meaningful benchmark that could reduce clinic congestion and improve patient compliance in real-world settings.
However, for now, retina specialists must continue to individualize care within the current range and await additional data that may support longer intervals in select populations.
Regeneron eyes broader flexibility
Despite this setback, Regeneron is not hitting any further pause on its blockbuster EYLEA HD. The company has already submitted another sBLA, aiming to move the drug into retinal vein occlusion (RVO treatment). This filing seeks:
- Approval for EYLEA HD to treat macular edema RVO
- Addition of every-4-week dosing as an option across currently approved indications
The FDA has set a target action date of August 19, 2025 for this pending application.
READ MORE: FDA Grants Priority Review to Eylea HD for Macular Edema Following RVO
Regeneron also continues to look towards the long-term extension phases of PULSAR and PHOTON, which may yield further insights on durability and outcomes beyond 96 weeks. If these later data sets confirm sustained efficacy and safety at extended intervals, a resubmission to the FDA could still be possible.
Additionally, Bayer, which co-develops aflibercept globally, has continued publishing and presenting clinical trial updates in peer-reviewed journals such as The Lancet, offering independent validation of the trial structure and outcomes.4,5
Editor’s Note: See Regeneron’s press release on the EYLEA HD extended interval CRL for more information. This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
References
1. Korobelnik JF, Heier JS, Khanani AM, et al. Two-year results of the PULSAR trial of aflibercept 8 mg with dosing up to every 16 weeks in neovascular age-related macular degeneration. Lancet. 2024;403(10431):1253-1264.
2. Busch C, da Cruz N, Ciulla TA, et al. Two-year results of the PHOTON trial of aflibercept 8 mg with dosing up to every 24 weeks in diabetic macular oedema. Lancet. 2024;403(10431):1265-1274.
3. American Journal of Managed Care. FDA delivers CRL for longer dosage intervals in aflibercept for DME, wet AMD. 2025. Available from: https://www.ajmc.com/view/fda-delivers-crl-for-longer-dosage-intervals-in-aflibercept-for-dme-wet-amd Accessed on April 22, 2025.
4. Lanzetta P, Korobelnik JF, Heier JS, et al; PULSAR Investigators. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet. 2024;403(10432):1141-1152.
5. Brown DM, Boyer DS, Do DV, et al; PHOTON Investigators. Intravitreal aflibercept 8 mg in diabetic macular oedema (PHOTON): 48-week results from a randomised, double-masked, non-inferiority, phase 2/3 trial. Lancet. 2024;403(10432):1153-1163.