New approval brings advanced retina diagnostics to Chinese ophthalmologists.
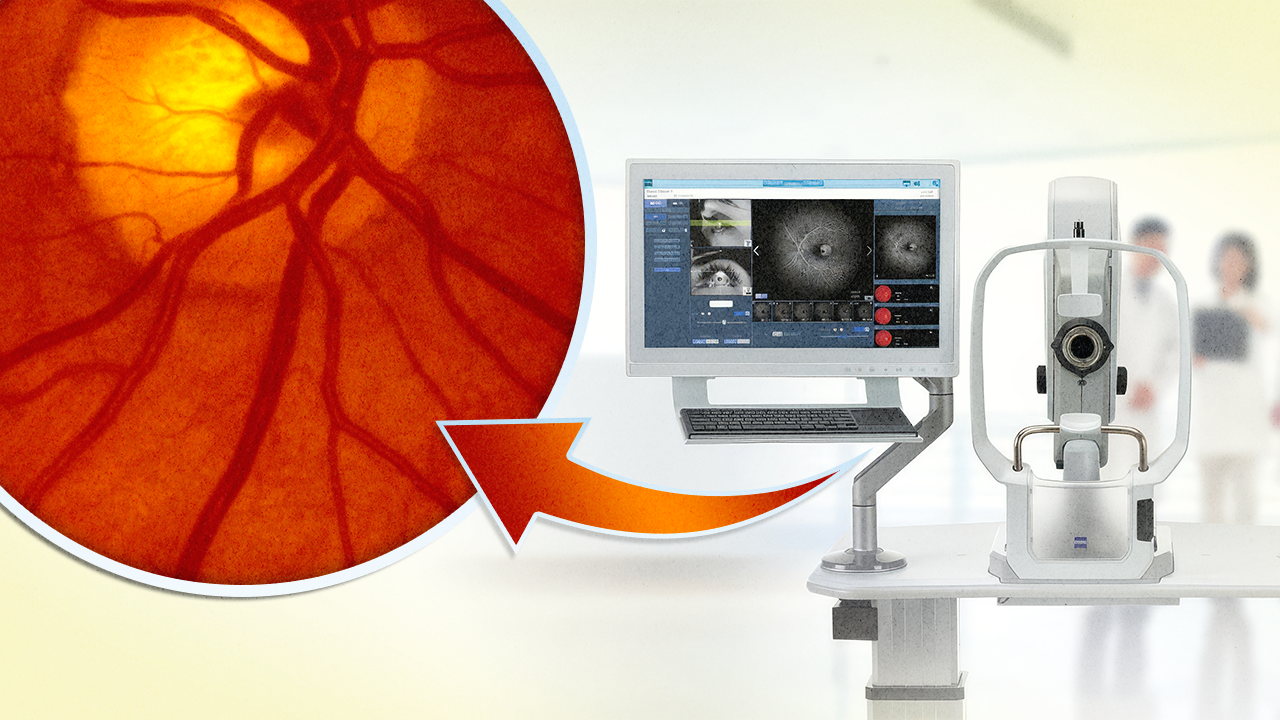
China’s retina specialists are getting a sharp new tool in their imaging arsenal. ZEISS Medical Technology (Jena, Germany) announced that its CLARUS 700 ultra-widefield retinal imaging system has received regulatory approval from the National Medical Products Administration (NMPA) in China.
The clearance is a big step for diagnostic precision, combining true-color fundus photography with high-resolution fluorescein angiography (FA), and wrapping it all into one AI-enhanced package.
READ MORE: Tackling Retinal Medicine’s Biggest Challenges With ZEISS
“ZEISS CLARUS 700 represents a major step forward in retinal imaging,” said Anuj Kalra, head of Chronic Disease Management at ZEISS, in the company’s official release.
What makes CLARUS 700 different?
There’s no shortage of fundus cameras on the market but CLARUS 700 stands out with a trifecta of true color, ultra-widefield imaging and high-resolution FA baked into a single platform. Unlike pseudo-color systems that use monochromatic channels, CLARUS captures high-resolution images in True Color, helping clinicians detect subtle pathology across the retina’s full landscape.
With a single 133° image (or 267° with montage), CLARUS 700 visualizes both central and peripheral retinal pathology in sharp detail. That includes peripheral ischemia, non-perfused zones and early signs of neovascularization that might otherwise go undetected.
According to ZEISS, the built-in fluorescein angiography mode offers quick, high-contrast capture of vascular leakage and perfusion status, which is critical for DR, retinal vein occlusions (RVO), and inflammatory conditions.
READ MORE: Adaptive Optics Enters the Clinical Arena at ARVO 2025
And because the system uses AI-enhanced optimization features like AutoBright and PrecisionFocus, images are adjusted automatically in real-time, cutting down on technician training time and giving ophthalmologists a clearer, faster diagnostic pathway.
CLARUS also includes:
- Live infrared preview, which allows visualization before capture
- QuickCompare feature for side-by-side review of pathology over time
- Auto-montaging that expands field of view
The True Color imaging format more closely resembles what clinicians see through direct ophthalmoscopy, meaning fewer surprises during diagnosis and treatment planning.
[VIDEO: First Experience with ZEISS CLARUS 700 with Dr. Abhinav & Dr. Ravinder https://www.youtube.com/watch?v=QgZn5tDv26o]Where diagnostic tech meets daily workflow
The CLARUS 700 is more of a productivity tool than a camera in the ZEISS Retina Workflow. Features like AutoBright, QuickCompare and PrecisionFocus support time-pressed clinics by minimizing image adjustment and accelerating the path from capture to clinical decision.
ZEISS has also integrated live infrared imaging for real-time visualization during image capture, making it easier to position patients and target pathology. Combined with FA capabilities, this setup promises comprehensive, color-faithful assessments across the full retina, even in patients with poor fixation or small pupils.
Maxwell Liu, head of Sales & Services for ZEISS Medical Technology China, noted the system’s relevance for the local market, saying, “It will provide unparalleled diagnostic precision for Chinese doctors and unprecedented comfort for their patients.”
Why this matters for China’s expanding retina care market
China is facing a sharp rise in diabetes and age-related eye diseases, with DR and RVO now leading causes of visual impairment.1 As patient volumes grow, so does the demand for faster, more accurate diagnostics, especially those that can reach the retinal periphery where early signs often hide.
According to Xiao et al., the CLARUS system showed moderate to substantial agreement with standard imaging protocols while capturing more peripheral pathology than five-field fundus photography or even other ultra-widefield devices.2 This expanded view can be critical for grading early DR and tailoring timely intervention.
READ MORE: Imaging Protocols and Best Practices to Optimize DR Treatment
The CLARUS 700’s true-color fidelity gives doctors a clearer picture of subtle changes in microaneurysms, hemorrhages and leakage, reducing diagnostic ambiguity and improving longitudinal monitoring. The Spotlight Case Compendium also highlighted real-world use cases where the system supported diagnoses ranging from ischemic retinopathies to complex AMD presentations.3
With the NMPA’s green light, the ZEISS CLARUS 700 enters one of the world’s largest and fastest-evolving ophthalmic markets. Its fusion of stats and software support aim to meet the growing demand in China for high-tech healthcare solutions.
Editor’s Note:
- Read the press release about the ZEISS CLARUS 700 approval for more details.
- This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
References
- Liu L, Liu LM, Hu YD, et al. Epidemic studies of diabetic retinopathy in China: a review. Int J Ophthalmol. 2011;4(6):670–672.
- Xiao Y, Dan H, Du X, et al. Assessment of early diabetic retinopathy severity using ultra-widefield Clarus versus conventional five-field and ultra-widefield Optos fundus imaging. Sci Rep. 2023;13:17131.
- Carl Zeiss Meditec, Inc. ZEISS Imaging Spotlight: Case compendium. 2020. Available from: https://www.zeiss.com/clarus