Medications meant to heal can harm, and an AAO 2025 Day 4 symposium revealed retinal toxicity’s hidden toll—and what docs can do about it.
Not all threats to vision come directly from disease.
On the final day of the American Academy of Ophthalmology Annual Meeting 2025 (AAO 2025), a packed symposium turned the microscope to an oft-overlooked culprit: the medications many patients take to stay alive (and have some illicit fun).
From cancer therapies to party drugs, six experts mapped the retinal damage lurking in medicine cabinets, emergency rooms and even nightclub corners—and the signs and biomarkers needed to preserve sight in some of our most vulnerable patients.
Miss me with the bullseye
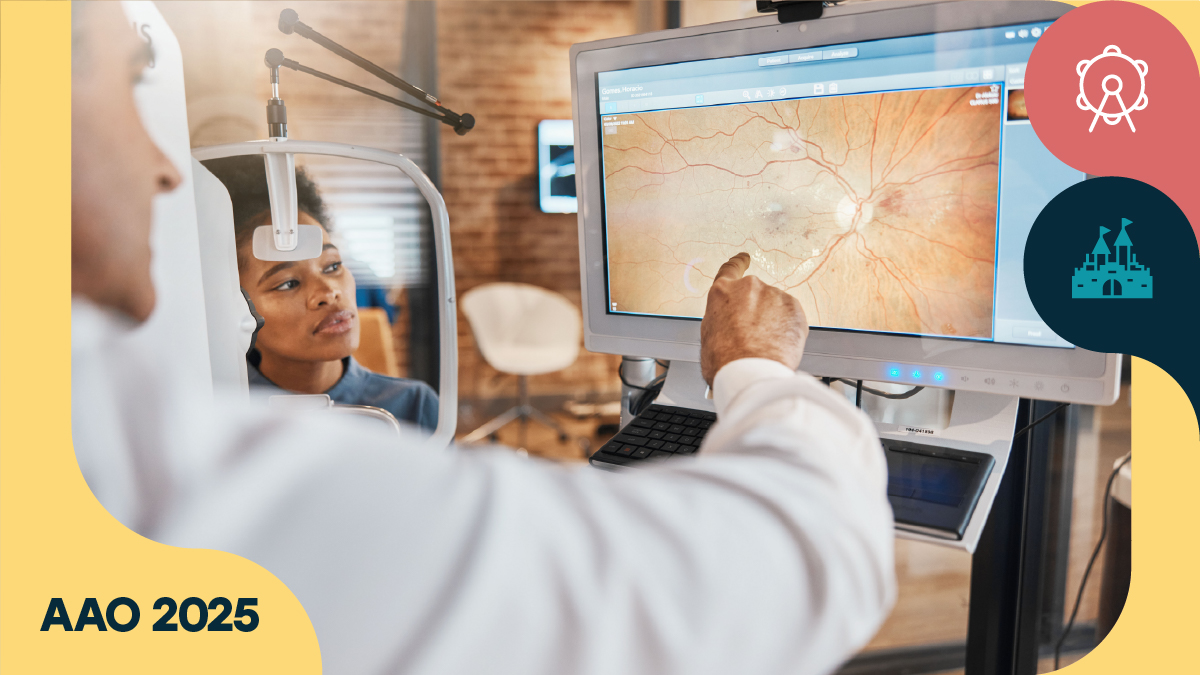
Dr. Amani Fawzi (USA) opened with a blunt warning about hydroxychloroquine screening. One that could spell helplessness for a patient’s vision.
“If you see a bullseye in a patient, they probably have been missed for a very long time,” Dr. Fawzi said. “We must strive to eliminate this bullseye maculopathy, because I think when we get there, it’s too late for the patients.”
Fortunately, modern imaging technology has the means to keep patients away from the cliff edge that a bullseye represents. According to Dr. Fawzi, the game has changed. OCT is now the workhorse—and a powerful one. Autofluorescence imaging, despite its growing popularity (and utility), can deceive clinicians in early cases.
And visual fields? Still useful when OCT findings remain questionable.
“If you see a patient where an external limiting membrane has disappeared, that’s really a patient you have to be vigilant about because they tend to progress even after you stop the medication,” Dr. Fawzi concluded, emphasizing a critical threshold.
The drug’s ionic trapping in lysosomes means toxicity can evolve long after discontinuation—a sobering reminder that stopping isn’t always stopping when the ball is rolling towards the bullseye.
READ MORE: Retina Roundup at AAO 2025: Fresh data, Familiar Challenges and the Future of Eye Care
Old drugs, new vigilance
The era of typical antipsychotics may be over, but its legacy could still linger in the retina.
Dr. Sharon Fekrat (USA) traced the evolution of antipsychotic medications and their retinal manifestations. Thioridazine, though discontinued, can cause toxicity years after patients stop taking it.
“I think more and more, we really need to be aware of what systemic medicines our patients are taking, especially if they don’t have one of the most common things that we see in our clinics,” Dr. Fekrat said.
While first-generation antipsychotics like thioridazine caused dose-dependent granular pigment retinopathy and nummular atrophy, newer atypical drugs like risperdal have proven far safer (for the retina, at least). Risperdal-related cystoid macular edema remains a rarity, mostly appearing in isolated case reports.
Dr. Fekrat’s concluding advice was twofold. First, look deep into patient histories, and start the investigations when antipsychotics like thioridazine pop up in a patient’s history.
And the second, though oft-repeated, is especially critical in the mental health landscape. “Working with the behavioral health and mental health colleagues is always key,” she said.
READ MORE: Adherence in 2025: How Close Are We to Finally Solving the Retinal Treatment Burden?
The PPS reckoning
Few toxicities have a discovery story quite like pentosan polysulfate (PPS) maculopathy. Dr. Nieraj Jain (USA) from Wills Eye Hospital recounted how a misdiagnosed “pattern dystrophy” led him to query his EMR—and uncover a hidden epidemic.
“If you’re thinking about any type of retinal degeneration, FAF imaging is your friend,” Dr. Jain said, describing how fundus autofluorescence made the distinctive maculopathy unmistakable.
But the real PPS gut punch? The relentless progression. “This condition is really bothersome to patients, and unfortunately, it just keeps getting worse,” he explained. Quality of life scores from his prospective study were among the lowest he’d ever seen.
His talk-ending call to action was direct. “I really encourage you to query your EHR. These are all patients that have been in our clinics for years, so we need to find them and get them off the drug.”
READ MORE: FDA Grants Rare Pediatric Disease Designation to Ocugen’s Gene Therapy for Stargardt Disease
Chemotherapy’s double edge
Cancer drugs save lives, but ophthalmic oncologist Dr. Jasmine Francis (USA) showed how they can complicate them, too. Her tour through oncologic pharmacology spanned generations, from traditional chemotherapy’s taxol-induced macular edema and tamoxifen’s crystalline deposits to the cutting edge of targeted therapies.
BRAF inhibitors, used for melanomas and thyroid cancers, can trigger bilateral anterior uveitis in about 5% of patients, occasionally progressing to cystoid macular edema. The mechanism remains unclear but the response to steroids is gratifying.
She also cited MAP kinase pathway inhibitors—FGFR, MEK and ERK blockers—where 15% of patients develop a distinctive retinopathy. Dr. Francis walked the audience through the OCT evolution: after just one day on the drug, the interdigitation and ellipsoid zones split apart. By day ten, fluid accumulates beneath the interdigitation zone. Then, remarkably, it resolves, even as patients continue treatment.
“This is intermittent, it’s temporary, and it’s reversible even when patients remain on drugs,” Dr. Francis reassured the audience. “You don’t necessarily have to stop patients who are on this drug.”
She offered another key pearl for FGFR inhibitors: Patients on these drugs can develop keratopathy, something easily missed if you’re focused solely on the posterior segment.
Immune checkpoint inhibitors—the PD-1, PD-L1 and CTLA-4 blockers revolutionizing cancer care—present a different challenge. About 1% of patients develop inflammation, but “literally any part of the eye can become inflamed,” Dr. Francis warned.
The presentation included striking examples of vitiligo developing on the fundus, progressive choroidal thinning and even choroidal nevi that evolved over time on checkpoint inhibitors. Patients can develop optic neuritis without other ocular signs, and distinguishing immunotherapy-induced inflammation from actual intraocular metastases becomes critical.
After her whirlwind tour through almost a dozen agents, her conclusion echoed Dr. Fekrat’s: Stay in touch with the patient’s oncologists, and stay vigilant.
Crystals in the crosshairs
Dr. Jaclyn Kovach (USA) delivered a visual tour of crystalline retinopathies via a guessing game with the rest of the presenters that left many of the biggest names in the space stumped. However, she emphasized one crucial principle above all others.
“I can’t stress enough the importance of taking a good medication history in our patients,” Dr. Kovach said, noting that supplements and recreational drugs often go unreported.
Tamoxifen emerged as particularly troublesome, causing CME, crystalline deposits and RPE changes that can mimic macular telangiectasia type 2. For symptomatic patients on secondary prevention, Dr. Kovach suggested a collaborative approach: “Symptomatic patients who take tamoxifen for secondary prevention could discuss with their oncology team possible drug discontinuation.”
READ MORE: A Wrinkle in Your Retina (and Macula!)
When recreation becomes retinopathy
Dr. William Mieler (USA) closed with the most sobering reality check about a decidedly non-sober topic: recreational drugs and their retinal toll.
Cannabis, legal in 40 states for recreational use and used by 60 million Americans as of 2022, presents an ongoing debate. “Is it harmful or beneficial? For the most part, that’s still being debated,” Dr. Mieler said, noting minimal visual symptoms despite some documented ERG changes.
Weed is not coffee, but even caffeine was on the hook. Dr. Mieler described “coffee and donut maculopathy”—acute macular neuroretinopathy triggered by excessive caffeine intake. And the stories he shared were often stranger than fiction. “A 48-year-old had some work to do and took three of these energy drinks back to back to back,” Dr. Mieler recounted, showing cringeworthy images of acute hemorrhagic changes and persistent scotomas.
Cocaine abuse painted an even grimmer picture: contaminant particles of substances commonly cut with the drug causing vascular occlusions, endophthalmitis from chronic injection and devastating retinal non-perfusion. With 1.5 to 2 million users and 16,000 annual deaths in the United States, the problem isn’t trivial.
Dr. Mieler’s closing observation captured one of the overarching themes of the session perfectly: the importance of thorough history checks and collaboration with other healthcare practitioners. “If you think it’s hard sometimes taking a history of known medicinal medications, trying to get people to admit what they take illicitly is something else.”
Editor’s Note: The American Academy of Ophthalmology Annual Meeting 2025 (AAO 2025) was held October 17-20, 2025, in Orlando, Florida. Reporting for this story took place during the event. This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.