Fewer injections, same outcomes? New PULSAR data suggest durability may be catching up in PCV.
In retina care, durability has long been the holy grail. A new post hoc subgroup analysis from the Phase III PULSAR randomized clinical trial suggests clinicians may be inching closer, at least for patients with polypoidal choroidal vasculopathy (PCV).
Published in JAMA Ophthalmology, the analysis found that aflibercept 8 mg delivered visual and anatomic outcomes comparable to aflibercept 2 mg in patients with PCV, while allowing dosing intervals of up to 16 weeks.*
READ MORE: Retina on the Ropes: Tackling the Toughest Debates in Medical Retina at APVRS 2025
Study design and patient population
The study was conducted as a post hoc subgroup analysis of the PULSAR randomized clinical trial, a Phase III study evaluating high-dose aflibercept in neovascular age-related macular degeneration (nAMD).
A total of 139 participants with indocyanine green angiography (ICGA)-confirmed PCV were included. Patients were enrolled from hospitals and clinics across 12 countries, with the mean age ranging from 72.2 to 73.2 years. Most of the patients were Asian (69.8%), reflecting higher prevalence of PCV in Asian populations. All participants were treatment-naive and followed through week 48.*
Treatment arms and dosing protocol
After three initial monthly loading doses, participants were randomly assigned in a 1:1:1 ratio to one of the three regimens: aflibercept 8 mg every 12 weeks, aflibercept 8 mg every 16 weeks, or aflibercept 2 mg every 8 weeks.
From week 16 onward, dosing intervals in the aflibercept 8 mg arms could be shortened if predefined disease activity criteria were met at prespecified visits. Interval extension was not permitted within the first year of the study. Participants assigned to aflibercept 2 mg followed a fixed dosing schedule of every eight weeks throughout the study period.
READ MORE: Innovation with Intention: Upcoming Retinal Disease Therapies on Spotlight at APVRS 2025
Visual acuity results
At week 48, visual outcomes were comparable across all treatment arms. Least-squares mean changes in best corrected visual acuity (BCVA) were:*
- +9.5 letters in the aflibercept 8 mg every 12 weeks group
- +8.4 letters in the aflibercept 8 mg every 16 weeks group
- +9.1 letters in the aflibercept 2 mg every 8 weeks group
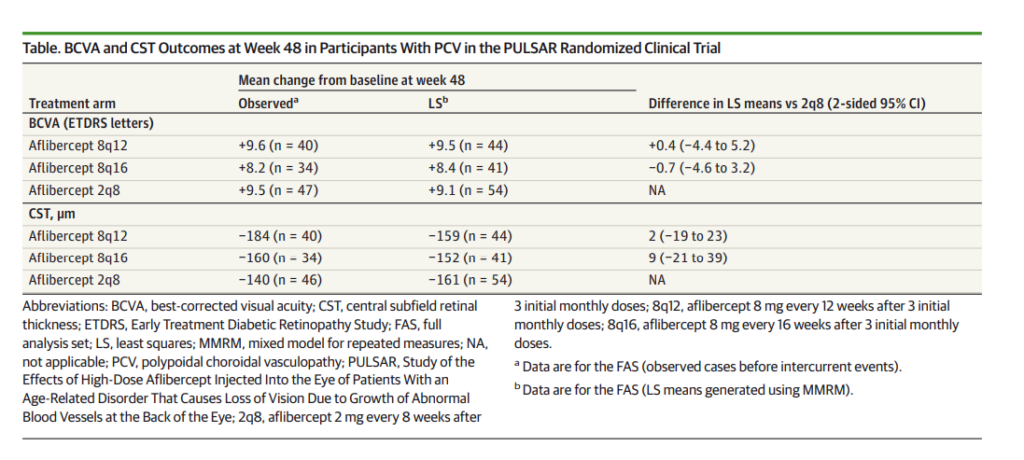
The study met its prespecified noninferiority criteria. The estimated difference in BCVA change was:*
- +0.4 (95% CI, −4.4 to 5.2) letters for aflibercept 8 mg every 12 weeks versus aflibercept 2 mg every 8 weeks
- −0.7 (95% CI, −4.6 to 3.2) letters for aflibercept 8mg every 16 weeks versus aflibercept 2 mg every 8 weeks.
Mean absolute BCVA at week 48 was approximately 20/50 across all treatment groups.*
READ MORE: Aviceda to Advance GA Drug into Phase III Trials Despite Phase IIb Primary Endpoint Miss
Anatomic outcomes and polyp activity
Anatomic outcomes closely mirrored the visual findings. Reductions in central subfield retinal thickness were observed across all three treatment arms.
At week 48, the observed mean CST change from baseline was:*
- −184 μm in the aflibercept 8 mg every 12 weeks group
- −160 μm in the aflibercept 8 mg every 16 weeks group
- −140 μm in the aflibercept 2 mg every 8 weeks group.
Least-squares mean CST changes were comparable between groups.*
Polyp outcomes were also similar. Among participants who completed week 48, polypoidal lesions were absent in:*
- 37% of patients treated with aflibercept 8 mg every 12 weeks
- 47% of those treated with aflibercept 8 mg every 16 weeks
- 38% of those treated with aflibercept 2 mg every 8 weeks.
Across treatment arms, 69% to 71% of participants no longer had active polypoidal lesions.*
Injection frequency and treatment durability
Injection frequency emerged as a key differentiator between treatment arms. Through week 48, 87% of participants treated with aflibercept 8 mg maintained dosing intervals of 12 weeks or longer.*
Participants who completed week 48 received a mean of:*
- 6.1 injections in the aflibercept 8 mg every 12 weeks group
- 5.1 injections in the aflibercept 8 mg every 16 weeks group
- 7.0 injections in the aflibercept 2 mg every 8 weeks group.
READ MORE: When Retina Meets Glaucoma at APVRS 2025
Safety and limitations
Safety outcomes were consistent across treatment groups. The authors reported similar safety profiles for aflibercept 8 mg and aflibercept 2 mg, with no new safety signals identified in the PCV subgroup.*
No cases of endophthalmitis or occlusive retinal vasculitis were reported. Serious ocular adverse events were rare, and most treatment-emergent adverse events were mild.
The authors acknowledged several limitations. As an exploratory post hoc subgroup analysis, the study was not designed to test hypotheses, and the relatively small sample size of 139 participants limits the generalizability of the findings.
In addition, the fixed eight-week dosing schedule in the aflibercept 2 mg arm constrained direct comparisons with extended-interval regimes for the standard dose.
The takeaway
This post hoc subgroup analysis of the Phase III PULSAR trial demonstrates that aflibercept 8 mg achieved visual acuity and anatomic outcomes comparable to aflibercept 2 mg in patients with PCV, while enabling dosing intervals of up to 16 weeks.
With fewer injections over 48 weeks and no new safety concerns identified, the findings point toward a more durable treatment approach that may ease clinic burden and treatment fatigue. For clinicians managing patients with PCV, the data suggests that maintaining outcomes may not always require more frequent visits. Sometimes, longer really does work just as well.
Editor’s Note: This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
*Lee WK, Wong TY, Chen SJ, et al. Aflibercept 8 mg in polypoidal choroidal vasculopathy post hoc analysis of the PULSAR randomized clinical trial. JAMA Ophthalmol. 2025;ePub ahead of print.
