Suprachoroidal shortcuts, refillable implants and even robots: pick your retinal route.
If intravitreal injections were a dinner party guest, they’d be the one who overstays their welcome. Necessary? Maybe…but exhausting for everyone.
On Day 2 of the 25th Congress of the European Society of Retina Specialists (EURETINA 2025), one symposium tackled that very problem, spotlighting ways to lighten the load for patients and clinicians alike. From refillable implants to creative routes through the eye’s hidden spaces, drug delivery is getting smarter, slicker and just a little more futuristic.
Port Delivery System
Prof. Aude Couturier (France) unveiled the kind of data every retina specialist dreams of: five years of durability from the Port Delivery System (Roche, Basel, Switzerland), the surgically implanted reservoir that slowly releases ranibizumab.
READ MORE: FDA Approves the First Anti-VEGF Port Delivery System for Diabetic Macular Edema
“We now have five years of clinical data on the PDS for nAMD [neovascular age-related macular degeneration] from the Archway and Portal trials, along with two years of data for DME [diabetic macular edema] and DR [diabetic retinopathy] from the Pagoda and Pavilion trials,” she reported.
The takeaway? Refills stretched to six or nine months with hardly anyone needing a top-up in between. Specifically, 98% of nAMD patients, 96% of DME patients and 100% of DR patients made it through their first interval without supplemental treatment.
Visual acuity stayed steady too, with more than half of nAMD patients maintaining 20/40 vision or better throughout.
Safety also matured with technique. Vitreous hemorrhage rates fell from nearly 50% in early trial days to just over 5% after adding laser photocoagulation to the choroid. Endophthalmitis dropped to under 1% with improved Tenon’s capsule dissection.

READ MORE: Bayer Notches Dual EYLEA 8mg Wins With China nAMD Approval and EU 6-Month Interval Extension
Looking ahead, Prof. Couturier pointed to new molecules like zifibancimig (DutaFab targeting Ang-2 and VEGF-A; Roche), currently in the BURGUNDY Phase I/II study, as potential partners for the PDS platform.
Subretinal delivery
Delivering therapies to the subretinal space isn’t exactly a walk in the park. It’s more like threading a needle while riding a unicycle.
That was the picture painted by Dr. Fanny Nerinckx (Belgium), who spotlighted both the pitfalls and progress of the transvitreal route, the go-to approach for gene therapies, stem cells and subretinal implants.
The old-fashioned way (manual subretinal injections) comes with plenty of headaches: tremor-induced retinotomies that stretch wider than intended, misplaced needles, shearing forces and the frustrating loss of precious payload to reflux.
“Studies have shown that up to 60% of the injected volume may reflux into the vitreous cavity,” Dr. Nerinckx said, underscoring just how leaky and inefficient the process can be.
Luckily, technology is starting to catch up. Automated injection systems, like the FDA-approved INCIO microinjection system (DORC Global, Zuidland, The Netherlands), now regulate pressure with the kind of consistency no human hand can guarantee.
Meanwhile, robotics is moving from science fiction to OR reality. “We performed a first-in-human clinical trial using the Acusurgical (Montpellier, France) Luca robot that allows bimanual surgery,” she reported. “When we think about robots, we think we have to freeze the instrument. But when performing a large bleb, you have to be dynamic and lift the subretinal needle as the bleb enlarges.”

READ MORE: Researchers Develop Head-Mounted Robotic System for Retinal Surgery
And while the tools are getting sharper, so is awareness of what happens after injection. Dr. Nerinckx stressed the need to monitor for inflammation and toxicity, pointing to recent findings on gene therapy-associated uveitis.1 “It is important to know because we have to give immunomodulating treatment to the patients pre- and postoperatively,” she said.
Optimizing bleb formation
Subretinal injections may look straightforward on paper (poke, inject, done), but anyone who’s tried knows the bleb has a mind of its own.
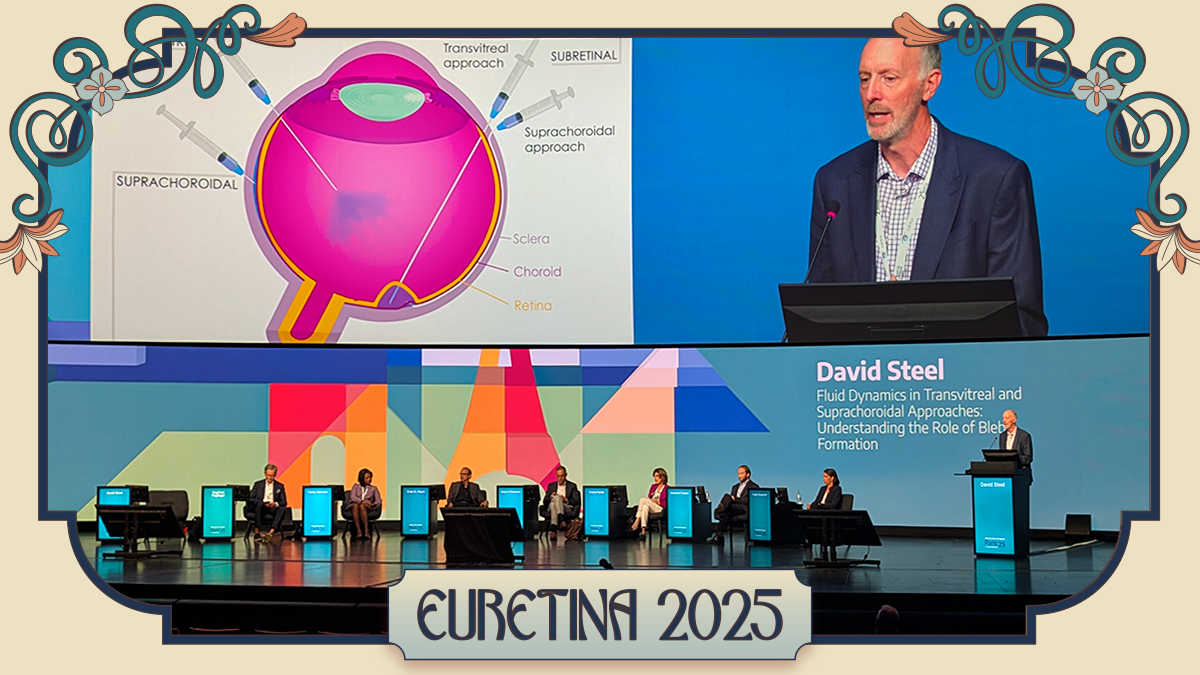
Prof. David Steel (UK) broke down the fluid dynamics of bleb formation with the precision of an engineer and the pragmatism of a surgeon who’s seen reflux ruin a good day.
“Retinal stress in a bleb is greater at its apex, which is typically near the retinotomy,” Prof. Steel explained. “If you inject an area of thin retina, you’re likely to have high stress at the injection point, which will stretch the injection point and lead to more reflux.”
He advised keeping intraocular pressure (IOP) low during injection, dialing the flow rate down to a gentle one to three microliters per second, and resisting the urge to go all-in with one massive bleb.
“If you spread the dose across multiple blebs, you not only reduce the height by 25% but also increase the area by 50% compared to one bleb,” he noted.

And when it comes to the fovea, Prof. Steel’s advice was clear: keep your distance. “You want the fovea to be in the most distal part of the bleb, not anywhere near the apex, to reduce stress on the fovea as it detaches.”
Suprachoroidal approaches
If the vitreous cavity is the main stage, the suprachoroidal space is like the VIP backstage lounge: harder to get into, but once you’re there, the access is unbeatable. Both Prof. Dominik Fischer (UK) and Prof. Siegfried Priglinger (Germany) pulled back the curtain on this narrow but promising therapeutic corridor between the sclera and choroid.
“Three arguments are put forward for suprachoroidal delivery,” Prof. Fischer explained. “First, if you could fill the suprachoroidal space completely, you could potentially reach 100% of the retina. Second, it’s an office-based procedure that avoids vitrectomy. Third, its proximity to the outer retina minimizes effects on the anterior segment.”
That kind of access comes with a growing toolbox: purpose-built microneedles with plastic stops for safe, office-based injections; transscleral injector systems in both micro- and macroneedle flavors; suprachoroidal catheterization; and even open surgical routes for trickier cases.
Prof. Priglinger’s team has gone one step further, successfully parking Ozurdex (AbbVie, Illinois, USA) implants in the suprachoroidal space for patients at high risk of anterior chamber migration—an elegant workaround for a tricky complication.
The safety record so far is encouraging. Across trial patients, there were no lens injuries, suprachoroidal hemorrhages or endophthalmitis, and just one case of retinal detachment.2
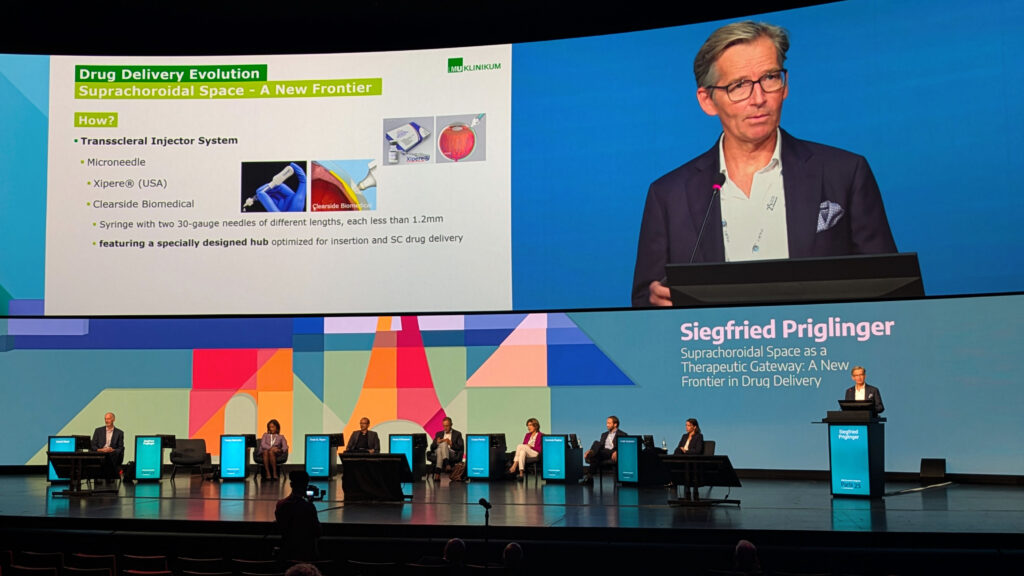
Better yet, Prof. Priglinger reported real-world gains: “In 72% of patients, we saw a reduction of central retinal thickness by 35%, and in responder patients, we saw a significant improvement in visual acuity,” he said.
Still, questions remain. How long will therapies last in this space, especially gene vectors that prefer a permanent address? Will filling the suprachoroidal space actually deliver the holy grail of 100% retinal coverage? Or will biology remind us who’s boss?
“Suprachoroidal injections are a welcome tool in our arsenal for retinal drug delivery, but [their] pros and cons must be considered in the context of which condition you treat, where you target and what is the payload,” Prof. Fischer concluded.
Transconjunctival suprachoroidal buckling
Who says you need a scleral buckle to fix a retinal tear? Certainly not Prof. Ehab El Rayes (Egypt), who is pushing the boundaries of retinal detachment repair with a decidedly less invasive route: transconjunctival suprachoroidal buckling.
The concept is as elegant as it is disruptive. Instead of sewing, tacking or otherwise wrangling the sclera, his approach uses a new device with a rounded, atraumatic needle tip to slip filler directly into the suprachoroidal space, all through the conjunctiva.

READ MORE: Latest Techniques for Retinal Detachment Success From APAO 2025
The filler of choice? Hyaluronic acid, which lingers a comfortable 12 to 14 months in the suprachoroidal pocket. He purported that, in a randomized trial, this method delivered a 91% single-injection success rate for repairing detachments, which certainly puts it in the “worth paying attention to” category.
“You don’t need a sclera to close a retinal tear,” Prof. El Rayes quipped, distilling the philosophy behind the technique.
He added that the procedure is not just minimally invasive but refreshingly straightforward. “It could be done using just an indirect ophthalmoscope, and it’s potentially an office-based procedure that avoids the problems of scleral buckling and vitrectomy,” Prof. El Rayes concluded.
The future of drug delivery
What emerged from this session is a field in motion: retinal drug delivery is shedding some of its needles-and-repeats monotony and embracing smarter, longer-lasting and less invasive options.
Whether it’s a refillable quietly doing its job for half a decade, a robot-guided bleb placed with surgeon-like finesse, or a microneedle tapping into the eye’s hidden highway, the future looks less like a treadmill of injections and more like a menu of tailored solutions.
Explore the latest retina breakthroughs in our daily EURETINA coverage.
Editor’s Note: The 25th EURETINA Congress is being held from 4-7 September, in Paris, France. Reporting for this story took place during the event. This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
References
- Purdy R, John M, Nerinckx F, et al. Gene therapy-associated uveitis (GTAU): Understanding and mitigating the adverse immune response in retinal gene therapy. Prog Retin Eye Res. 2025;106:101354.
- Asani B, Kruse F, Priglinger SG, et al. Suprachoroidal implantation of corticosteroid slow release implants for the treatment of cystoid macular edema. Scientific Reports. 2025;15:20166