jCyte turns up the dose, chasing broader answers for a disease with few options.
A higher dose, a broader target and a rare disease still lacking options. jCyte Inc. (California, USA) has dosed its first patients in the JC02-88 Phase II trial, testing an 8.8 million cell intravitreal injection of jCell (famzeretcel) in adults with retinitis pigmentosa (RP).
The dose, about 50% higher than prior trials, marks a push to explore the full therapeutic potential of jCell. Up to 60 participants will receive either the cell therapy or a sham injection, with outcomes tracked for safety, visual function and quality-of-life over six months.
“The initiation of patient dosing in this trial marks an important milestone in our mission to bring a breakthrough treatment to the majority of RP patients who currently have limited treatment options,” said John Sholar, CEO of jCyte.
READ MORE: FDA Grants Fast Track Designation to Oral Treatment for Retinitis Pigmentosa
Widening the window for intervention
jCell is an allogeneic retinal progenitor cell therapy delivered by intravitreal injection. Instead of fixing single-gene defects, the therapy works by releasing neurotrophic factors that help preserve surviving photoreceptors.
Key attributes of jCell include:
- Genotype-independent mechanism (unlike gene therapy)
- Minimally invasive intravitreal delivery
- No immunosuppression required
- Potential scalability for other retinal indications
Rather than targeting specific mutations, the therapy takes a broader swing at disease progression, aiming to intervene while photoreceptors are still viable.
“This is an exciting development for the RP community,” said Dr. Paul Sieving, former director of the U.S. National Eye Institute. “I am eager to see how this promising therapy advances toward providing a novel cell-therapy treatment for a patient population with vast unmet need.”
Dr. Henry Klassen, jCyte’s co-founder and president, added that this milestone reflects the team’s long-standing commitment to moving jCell toward approval. “Our team’s dedication and perseverance have brought us to this point, and we remain committed to delivering a therapy that can make a meaningful difference in patients’ lives,” he said.
READ MORE: Nanoscope Therapeutics to Submit BLA for Retinitis Pigmentosa Gene Therapy Candidate
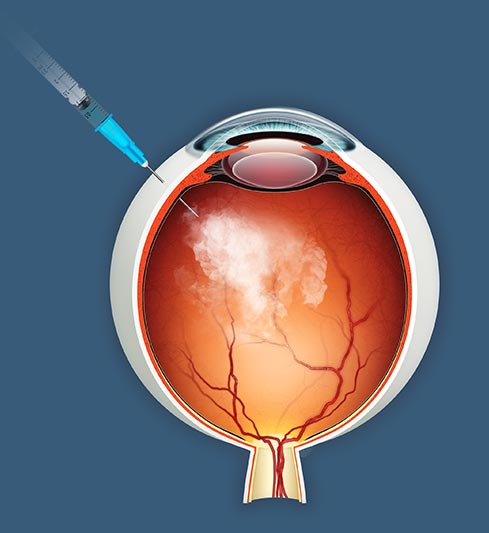
Beyond the gene and into the field
With no FDA-approved treatments available for the vast majority of RP patients, jCell sidesteps the limits of mutation-matched therapies, opening the door to broader applications in retinal disease.
The therapy has already received Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA, reflecting its potential to address a serious condition and its preliminary clinical promise.
The six-month, randomized JC02-88 trial is being conducted at the Gavin Herbert Eye Institute in Irvine, California, with support from partners including the University of California, Irvine, Good Manufacturing Practice Facility, Lexitas and the California Institute for Regenerative Medicine.
A long-term extension study will follow, giving participants who initially received sham treatment the opportunity to cross over to jCell therapy.
READ MORE: NIH-Funded Study Tests PEDF Peptide Eye Drops for Retinitis Pigmentosa and AMD
Trial enrollment and patient eligibility
JC02-88 is currently recruiting patients in Southern California. Key eligibility criteria include:
- Age 18 to 60
- Diagnosis of RP (any genetic subtype)
- Vision between 20/80 and 20/800 in the study eye
- Central visual field diameter ≥8°
- No recent participation in other trials (with some exceptions)
- Commitment to attend study visits in Irvine, CA (travel support provided)
More information and enrollment details are available at www.jcyte.com/JC02-88-study.
Editor’s Note: Read the official press release: jCyte Inc. Announces First Patients Treated in JC02-88 Trial for Retinitis Pigmentosa. This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.