At EURETINA 2025, five talks revealed how microglia, ischemia and vessel caliber signal risk long before vision fades.
The Grand Amphitheater of Palais des Congrès in Paris was the arena for a session devoted to diabetic retinopathy (DR) on Day 2 of the 25th Congress of the European Society of Retina Specialists (EURETINA 2025).
Guided by Prof. Edoardo Midena (Italy) and Prof. Dr. Reinier Schlingemann (Netherlands), five speakers tackled the condition from multiple vantage points spanning cellular inflammation, ischemic burden, structure-function correlation, AI-guided phenotyping and vascular signatures.
What emerged was a portrait of DR as a disease of early warning signs: quiet structural shifts that accumulate, interact and ultimately shape visual fate. It was science with urgency, casting light not just on where the retina stands today, but how the future of management might be rewritten.
READ MORE: Is This Retinal Oximeter The Next Step for Clinical Oculomics?
Zooming in on microglia’s role
Kicking off Friday morning’s session, Dr. Andreas Pollreisz (Austria) took the Grand Amphitheater deep into the retinal microenvironment where microglia, the immune sentinels of the retina, may be sounding early alarms in disease progression.
Using adaptive optics optical coherence tomography (AO-OCT), his team achieved volumetric, in vivo imaging of individual microglia with micrometer precision. The prototype system provided 10 times the resolution of conventional OCT, enabling real-time visualization of ramified (resting) versus activated microglia.
In diabetic eyes, there was a striking increase in activated microglia, particularly near the fovea, closely linked to other hallmarks such as microaneurysms, hard exudates and hyperreflective foci under 30 μm.

READ MORE: Intalight’s DREAM OCT Platform Cleared for EU Market with CE Mark
“Adaptive optics OCT allows the visualization of contrast structural details in diabetic eyes over time which are undetected with conventional OCT imaging,” Dr. Pollreisz explained.
“Through increased imaging resolution it allows in vivo retinal microglia sense, their retinal location, inter-retinal location and their activation states in humans.”
Dr. Pollreisz stressed that AO-OCT not only sharpens disease characterization at the cellular level, but also opens doors for monitoring early progression and therapeutic response. While imaging deeper retinal layers remains challenging, refinements in dual-signal resolution may soon widen this window into DR’s earliest inflammatory footprint.
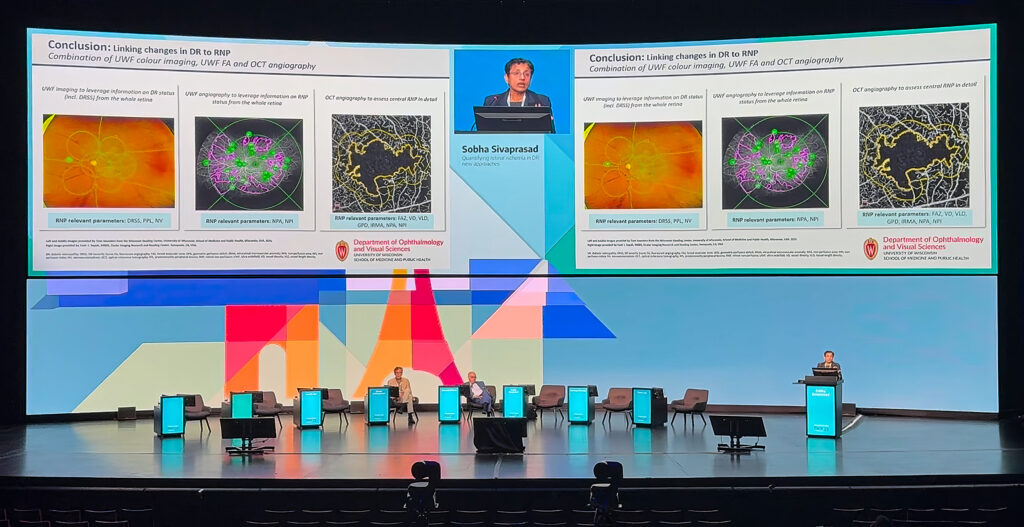
Measuring ischemia, still unfinished
Prof. Sobha Sivaprasad (UK) delivered a sobering update on the challenge of retinal ischemia measurement in DR, reminding the audience that a true gold standard is still out of reach.
Drawing on insights from the INSPIRE study and trials such as the Rho-PDE inhibitor (PER-1) and CRIMSON, she outlined how current tools often fall short.
While Diabetic Retinopathy Severity Scale (DRSS) scores can align with nonperfusion at a population level, the variability between individual patients is striking. Moreover, anti-VEGF therapy can improve DRSS scores without necessarily improving perfusion, creating a dissociation between clinical grading and vascular reality.
“We actually do not have any accurate methods in quantifying retinal ischemia at present,” Prof. Sivaprasad noted, emphasizing the mismatch between scoring systems and perfusion status.
READ MORE: FDA Approves Roche’s Susvimo for Diabetic Retinopathy
Conventional imaging like fundus photography, ischemic index mapping and even widefield angiography struggles with reproducibility, particularly at the periphery. Automated segmentation and AI-based analysis may improve matters, but variability is still apparent.
Prof. Sivaprasad pointed to emerging tools such as macular ischemic indices, OCT-A and flicker ERG, while stressing that central ischemia does not reliably predict peripheral involvement. For now, she argued, composite biomarkers will be essential for future trials, as demonstrated in early anti-SEMA3A studies. Until then, ischemia remains an elusive and fuzzy endpoint.
Mapping structure to function
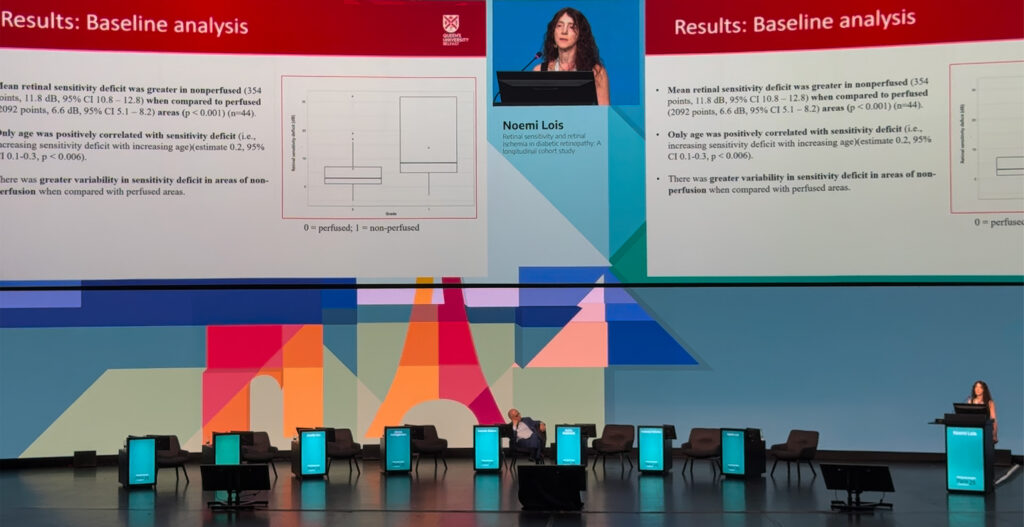
Prof. Noemi Lois (UK) presented findings from a rigorous two-year longitudinal study that probed the complex relationship between retinal ischemia and retinal sensitivity in DR.
Unlike earlier investigations limited to the macula or short follow-up, her team overlaid microperimetry data onto fluorescein angiograms across the full posterior pole, mapping point-by-point correlations between structure and function.
READ MORE: Visualizing the Retinal Vasculature
The results revealed a strong association that’s still far from perfect. Non-perfused regions generally displayed reduced sensitivity, yet many perfused zones still showed functional deficits, and some ischemic areas retained normal vision.
“We found a strong correlation between perfusion status and reference sensitivity, but we found sensitivity low in some perfused areas and normal sensitivity in some non-perfused areas,” Prof. Lois explained.
Approximately half of these anomalies could be attributed to factors such as overlapping vasculature or adjacency to ischemic zones. However, the remaining unexplained cases pointed to underlying mechanisms beyond visible perfusion status.
Importantly, Prof. Lois’s team found that retinal sensitivity declined gradually over time even in perfused regions, underscoring the subtle but significant functional impact of DR progression.
Her message was that vascular imaging and functional testing tell overlapping yet distinct stories. Both are essential for clinical management and for evaluating emerging therapies targeting neurodegeneration or reperfusion.
AI as the retinal refiner in DME
Diabetic macular edema (DME) is not one disease, but a spectrum of phenotypes and treating it as a single entity is a recipe for suboptimal outcomes. That was the message from Prof. Edoardo Midena (Italy), who opened his talk with the stark statistic that around 40% of patients fail to respond to anti-VEGF therapy after one year. The reason, he argued, is an overreliance on central subfield thickness, a crude measure that fails to capture the nuances of disease biology.
To address this, Prof. Midena’s team has developed an AI algorithm capable of segmenting and quantifying key OCT biomarkers, including intraretinal and subretinal fluid, ellipsoid zone integrity and hyperreflective foci. “The concept that we need to go to precision medicine to decide which kind of DME [we have] for this particular patient, [and] which is the best treatment,” he explained, “I can monitor this one according to the use of modern science and the technology.”

By consistently quantifying these biomarkers, the AI system enables clinicians to phenotype patients and guide therapy accordingly. In a dataset of more than 2,000 eyes, outcomes varied significantly by biomarker profile, pointing the way toward tailored, profile-driven DME management that anticipates recurrence rather than reacting to it.
READ MORE: Oral Lamivudine Shows Vision Gains in DME Patients in Early Clinical Trial
Reading between the vessels in PDR treatment
Dr. Jennifer Sun (USA) analyzed a post hoc dataset of the DRCR Retina Network’s Protocol S, asking a deceptively simple question: do panretinal photocoagulation (PRP) and anti-VEGF therapy leave different long-term signatures on the retinal vasculature?
Her team applied Integrated Vessel Analysis Software (IVAN) to high-quality fundus photographs from 107 eyes, measuring central retinal artery and vein equivalents (CRAE and CRVE). The results revealed early divergence. CRAE thinned faster in PRP-treated eyes during the first two years, while ranibizumab-treated eyes showed slower changes but gradually caught up by year five. CRVE shifts emerged later but ultimately showed greater thinning in the PRP group.
Importantly, vessel caliber changes were not just cosmetic. Greater thinning correlated with increased visual field loss, hinting at a possible structure-function link. “These results suggest that the two treatments may result in different rates of change in vascular caliber over time,” Dr. Sun explained, “which may need to be taken into account in future analyses of vascular caliber and treatment.”
While exploratory and limited by image availability, the findings hinted at intriguing possibilities. Vessel caliber may emerge as a biomarker not only for tracking treatment effects, but also for predicting long-term visual outcomes in proliferative DR.

READ MORE: Seeing the Unseen: Adaptive Optics Enters the Clinical Arena
Reading early signals
While no single biomarker or imaging modality can capture the full complexity of diabetic retinopathy, this session demonstrated how close the field is getting. From microglia and ischemia to structure-function mismatches, vessel caliber and AI-enabled prediction, each study pointed toward earlier, more nuanced detection and raised critical questions about structural change versus functional outcomes.
As the focus shifts from late intervention to true preservation, the tools of tomorrow may be those that can interpret the retina’s subtle cues long before vision fades. As EURETINA 2025 showed, the retina whispers its warnings early. The task ahead, therefore, is learning how to listen.
Explore the latest retina breakthroughs in our daily EURETINA coverage.
Editor’s Note: The 25th EURETINA Congress is being held from 4-7 September, in Paris, France. Reporting for this story took place during the event. This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.