FDA Approves Roche’s Susvimo for Diabetic Retinopathy
Genentech/Roche’s sustained release anti-VEGF eye implant provides a treatment option with reduced frequency for diabetic retinopathy. Sustained release anti-VEGF is…
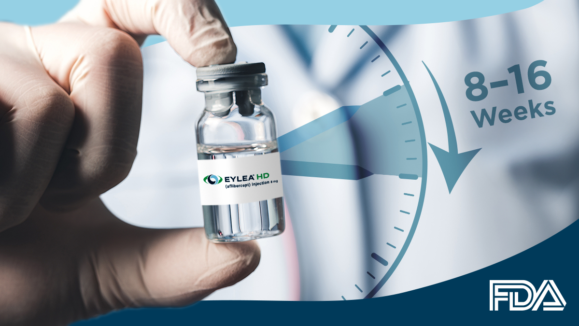
FDA Rejects Regeneron’s sBLA to Extend EYLEA HD Dosing Interval
Regeneron’s proposal to lengthen EYLEA HD dosing intervals to 24 weeks hits a CRL roadblock. The wait for extended treatment…
latest posts