We bring our EURETINA 2025 coverage out with a bang on Day 3 with inherited retinal disease updates, keynote lectures galore and more.
The final stretch of the 25th Congress of the European Society of Retina Specialists (EURETINA 2025) arrived with all the flair you’d expect from Paris—equal parts intellectual rigor and the latest innovations, all laced with the City of Light’s irresistible charm.
Retinal science has been in motion across these three days of the conference, and the Congress’ record attendance (11,000+ this year) has been a testament to the excitement around retinal medicine.
Our Day 3 coverage saw everything from gene therapy breakthroughs to surgical masterclasses, capped by a buzz we haven’t seen in retinal medicine for a very long time. Plateaus in traditional therapies like anti-VEGF are being pushed through, barriers to orphan disease treatment broken—and we were there to capture it all on our final day of coverage.
Session highlights
Our inherited retinal disease therapy (IRD) highlight session delivered a tour de force of scientific progress that we’ve grown used to at EURETINA 2025. From CRISPR gene editing to cell-based therapies, eight speakers charted a course through the most innovative approaches to conditions once considered untreatable.
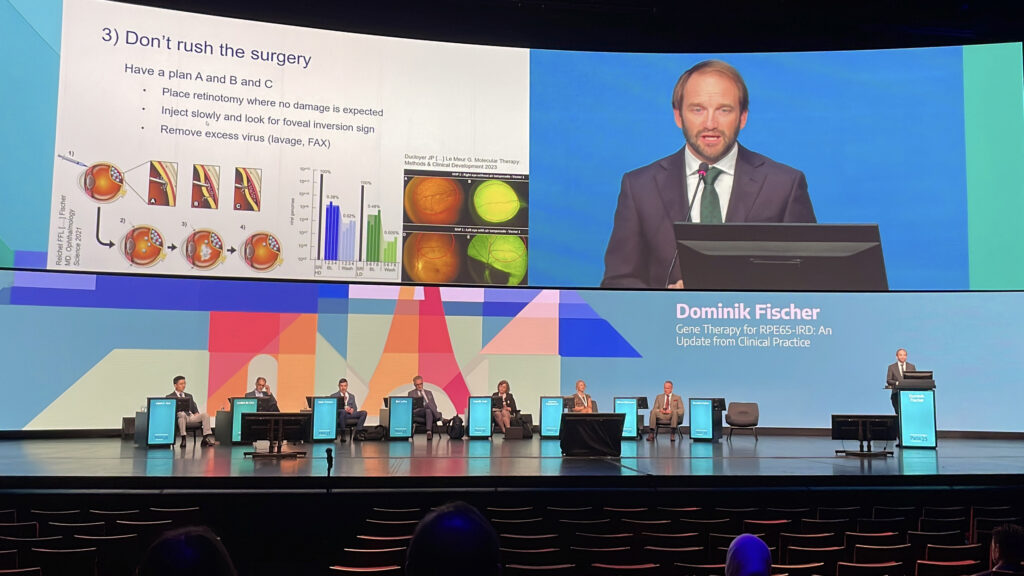
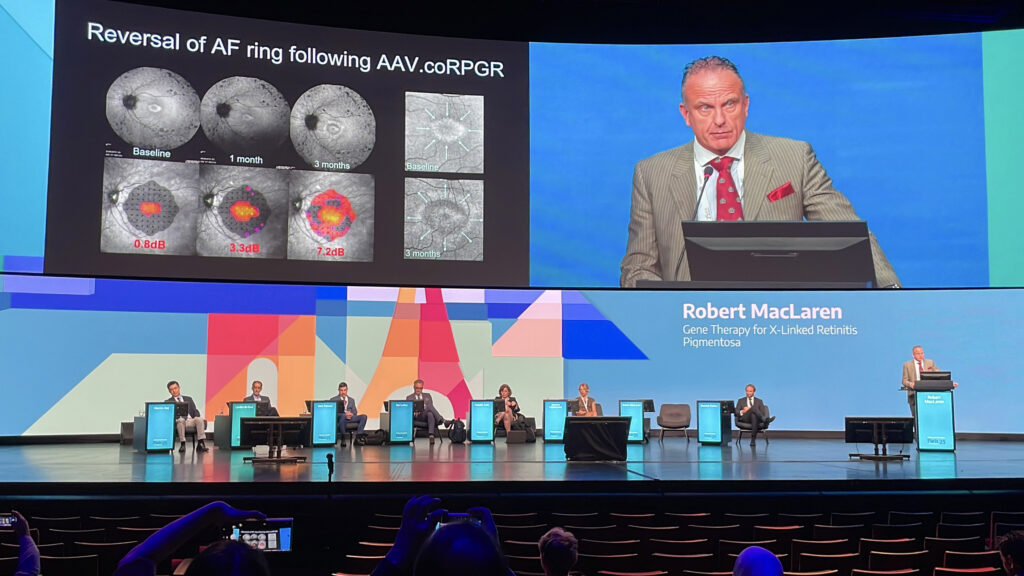
Prof. Dominik Fischer (Germany) opened with pragmatic real-world advice on RPE65 gene therapy, while Prof. Robert MacLaren (United Kingdom) presented compelling evidence of anatomical reversal in X-linked retinitis pigmentosa. Click to discover how rare diseases are becoming anything but rare to treat.
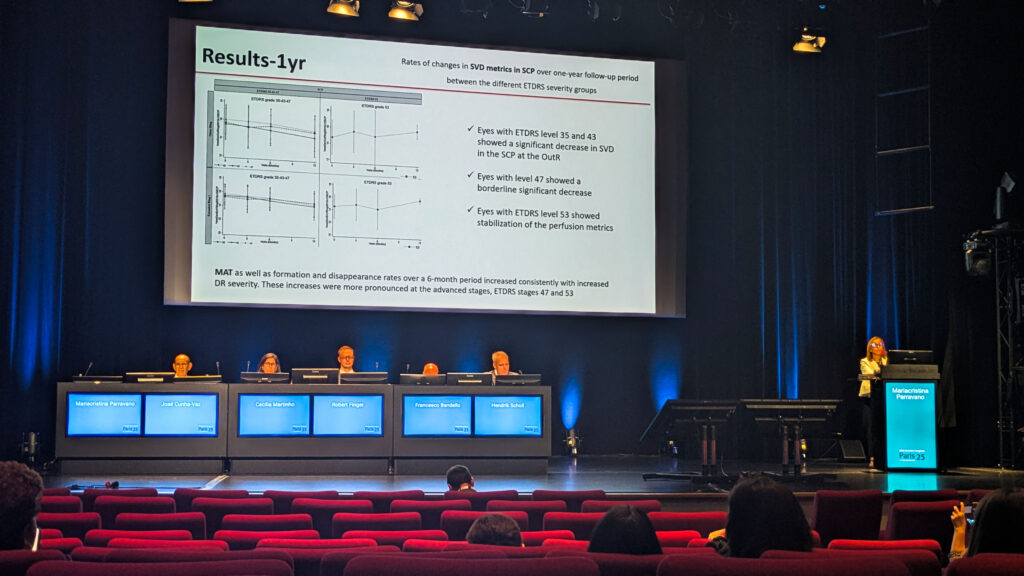
The EVICR.net symposium proved that European research networks know how to connect the dots—literally and figuratively. Dr. Mariacristina Parravano (Italy) shared fresh insights from the CHART study on diabetic retinopathy biomarkers, while Dr. Ines Pereira Marques (Portugal) revealed intriguing links between retinal changes and cognitive decline, reinforcing the idea that the eye truly is a window to the brain.

The session also unveiled exciting transatlantic collaborations between EVICR.net and the U.S.-based DRCR Network. Click to uncover what oculomonic secrets the retina is whispering about systemic health.
The prestigious Gisbert Richard and Kreissig Award lectures delivered surgical wisdom with the kind of authority that only comes from decades in the operating room. In her Kreissig Award lecture, Prof. Maria Berrocal (Puerto Rico) urged the field not to abandon time-tested techniques, making a compelling case for scleral buckling and laser photocoagulation in today’s era of injection enthusiasm.

Prof. Jean-François Korobelnik’s (France) methodical approach to intraocular foreign body removal demonstrated that surgical excellence lies not in flash, but in disciplined technique and careful judgment.
Click now for the full details…and a parade of pearls.
Industry updates
It’s retina’s week, after all, and research updates abound in the lead-up to EURETINA 2025.
Roche (Booth #2.B30, 2.B40) remained in the spotlight at EURETINA 2025 with the announcement that it has received the EU CE mark for its Port Delivery Platform with Susvimo. Marketed in the EU as Contivue®, the platform includes the ocular implant for Susvimo delivery along with four ancillary devices designed for filling, insertion, refilling and—if needed—removal of the implant. Susvimo® (ranibizumab injection, 100 mg/mL) is currently under review by the European Medicines Agency (EMA) for the treatment of nAMD. Offering immediate and predictable durability, Contivue with Susvimo provides continuous delivery of a customized ranibizumab formulation directly into the eye.
“The seven-year results from the LADDER study powerfully demonstrate the long-term outcomes delivered by Contivue with Susvimo,” said study investigator Dr. Carl C. Awh of Tennessee Retina (USA) in a press statement. “For patients with nAMD, its sustained delivery may offer superior visual outcomes compared to the well-documented average decline associated with long-term intravitreal injections.”
Meanwhile, Ashvattha Therapeutics (California, United States) reported positive topline results from its Phase II trial of migaldendranib (MGB) in diabetic macular edema (DME) and neovascular AMD (nAMD) at EURETINA 2025. The 40-week multicenter study evaluated subcutaneous MGB—a VEGF receptor tyrosine kinase inhibitor linked to a hydroxyl dendrimer—designed to cross the blood-retinal barrier and selectively target activated retinal cells.
“Throughout the 40-week trial, MGB was well tolerated and showed improvements in both vision and retinal anatomy, while significantly reducing the need for supplemental intravitreal injections in patients with active nAMD and DME,” said Dr. Arshad M. Khanani, managing partner and director of clinical research at Sierra Eye Associates (Nevada, United States) in a press statement.
“A once-monthly subcutaneous injection that delivers a bilateral therapeutic effect marks an important advance for patients with vision-threatening retinal diseases, with the potential to improve real-world outcomes and quality of life,” he added.
On the other hand, Valitor (Hafnarfjordur, Iceland), a biotechnology company developing next-generation ophthalmic medicines, presented new data on VLTR-559, its long-acting anti-VEGF biologic for wet age-related macular degeneration (AMD), at the Ophthalmology Futures Retina Forum (3 September, Pavillon d’Armenonville, Paris). In a non-human primate (NHP) study, VLTR-559 was well tolerated at the anticipated clinical dose and demonstrated a safety profile comparable to currently approved short-acting anti-VEGF therapies. Designed to enable a simplified “treat-and-release” regimen, VLTR-559 aims to ease disease management for both patients and physicians.
“A well-tolerated and potent anti-VEGF therapy administered only twice a year has the potential to improve long-term outcomes for patients,” said Wesley Jackson, PhD, President and CSO of Valitor. “Additionally, extending dosing intervals could lower clinical costs by reducing the number of visits required with first-generation therapies.”
These latest in vivo safety findings build on a series of encouraging results, highlighting VLTR-559’s potential to significantly reshape wet AMD treatment.
Until next year…
With that, the curtain falls on another remarkable EURETINA congress. As per usual, we’re left with more than just scientific updates—what’s possible when brilliant minds from around the world converge on the retinal challenges that matter most.

If you loved our coverage, the show rolls on to Copenhagen next week for some anterior segment action at the 43rd Congress of the European Society of Cataract and Refractive Surgeons (ESCRS 2025). E-join us in Denmark as we bring you the latest from the world’s marquee cataract and refractive meeting. See you there!
Editor’s Note: The 25th EURETINA Congress was held from 4-7 September, in Paris, France. Reporting for this story took place during the event. This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
