From tiny vessels to big ideas, EVICR.net is decoding the retina’s secret language.
One Day 3 session at the 25th Congress of the European Society of Retina Specialists (EURETINA 2025) was all about putting two and two together: from retina to brain, from Europe to the United States, and from early-stage disease to novel therapies. The European Vision Clinical Research Network (EVICR.net) symposium on diabetes and vascular disease proved that retinal research is about spotting patterns, predicting progression and, sometimes, uncovering secrets from the retina.
New NPDR insights and biomarkers
Diabetic retinopathy (DR) isn’t just about counting microaneurysms anymore. It’s about spotting patterns before the plot thickens and vision is irretrievably lost.
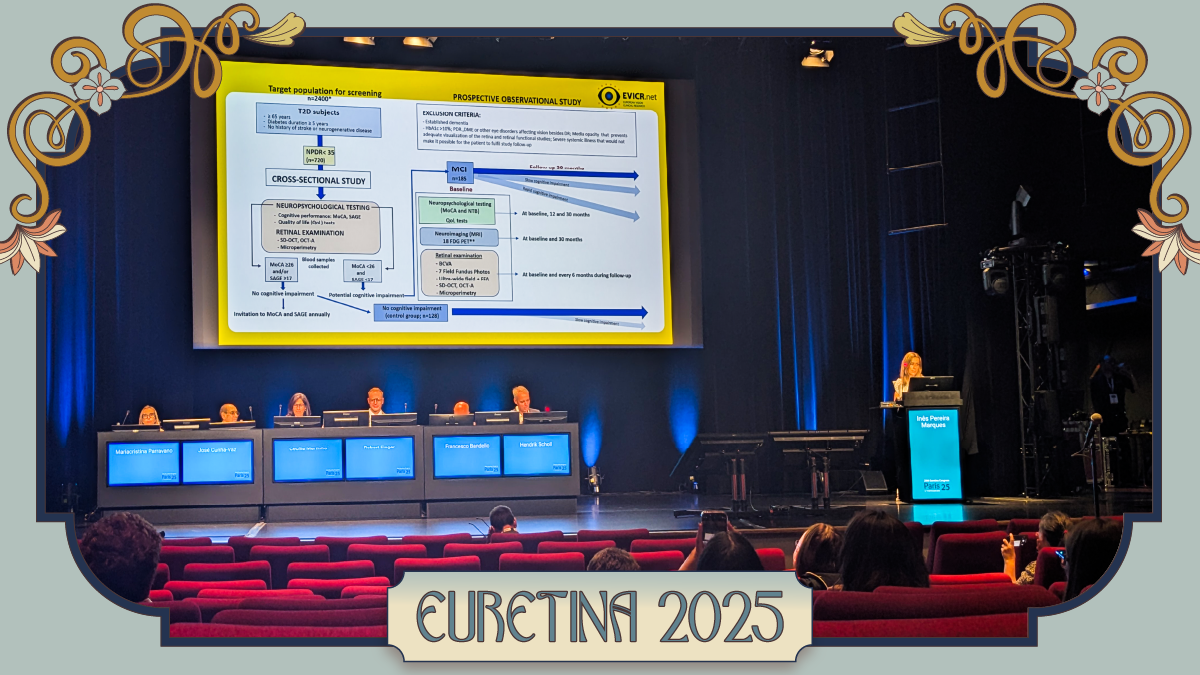
Dr. Mariacristina Parravano (Italy) shared fresh results from the CHART study, a five-center, pan-European project aiming to crack the biomarker code in non-proliferative diabetic retinopathy (NPDR) with non-invasive imaging.
“We were able to include 202 eyes from patients with type 2 diabetes with different severity stages of non-proliferative diabetic retinopathy,” she explained. To do the job, they reached for the heavy hitters of modern retinal imaging: 7-field color fundus photography, spectral domain optical coherence tomography (SD-OCT) and OCT angiography (OCT-A).
READ MORE: Seeing the Unseen: Adaptive Optics Enters the Clinical Arena
The payoff came in a key insight. “Measuring rates of progression in microvascular changes instead of absolute values in skeletonized vessel density and perfusion density allows more consistent and reliable monitoring of diabetic retinopathy progression,” noted Dr. Parravano. In other words, it’s less about the static numbers and more about watching the trend lines.

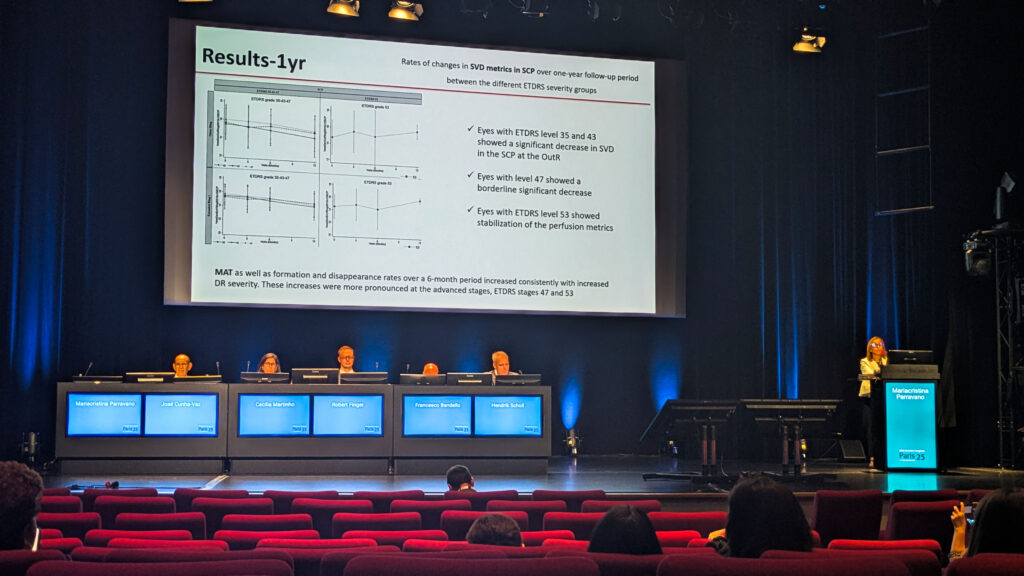
And those lines tell a story. According to Dr. Parravano, NPDR tends to move through a hypoperfusion stage, followed by a hyperperfusion stage as the disease advances.
When the retina talks
If you think the eye doesn’t gossip, think again. Turns out the retina may be spilling secrets about cognitive decline.
Dr. Ines Pereira Marques (Portugal) offered a glimpse into the RECOGNISED study, a cross-Europe effort to untangle how diabetic retinopathy and cognitive decline might be two sides of the same coin.
“With this study, we want to investigate the common mechanisms involved in pathogenesis of diabetic retinopathy and cognitive impairment in patients with type 2 diabetes,” she said. “We also want to find some tools for identifying individuals with type 2 diabetes at high risk of developing cognitive decline based on retina imaging.”
READ MORE: The Retina’s Early Warnings
The scope was impressive: 510 patients recruited at 11 sites across seven European countries, with 318 screened for longitudinal follow-up. Early cross-sectional results are already hinting at a telling link. Patients with mild cognitive impairment (MCI) had lower retinal sensitivity and less stable gaze fixation (subtle slips in function) even though their OCT structural parameters looked virtually identical.
Add in the electrophysiology results—higher implicit timing and lower amplitude flash response—and the retina looks like it’s whispering trouble long before the brain shows it.

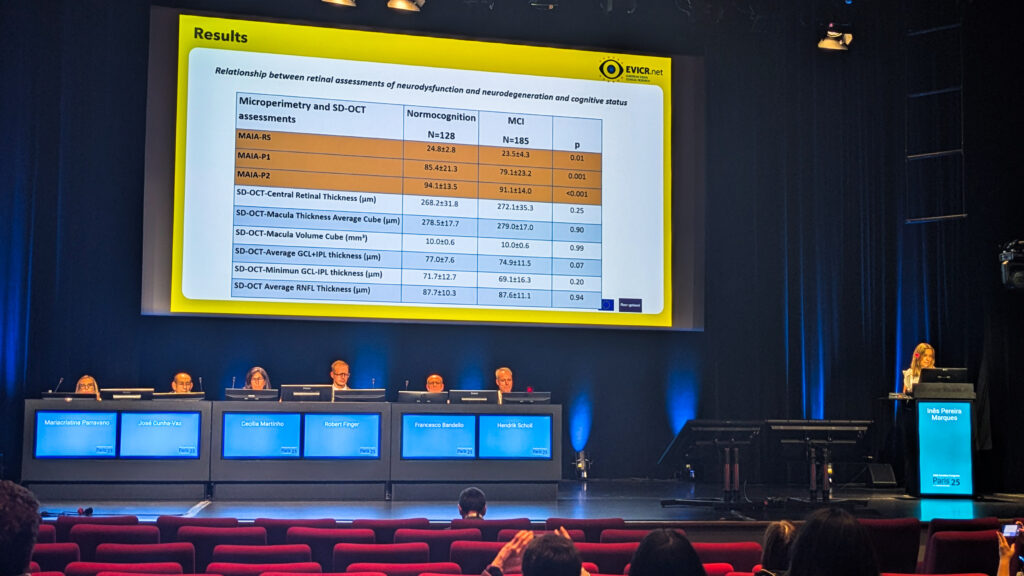
Taken together, the findings suggest the retina might be pulling double duty as both a window to systemic health and an early alarm bell for cognitive decline. As Dr. Marques concluded, they support “the use of microperimetry and RETeval [LKC Technologies; Maryland, USA] in combination with a single clinical variable as a new strategy to identify diabetes-associated cognitive impairment.”
A pond-crossing DR research alliance
Forget long flights and jet lag. Europe and the U.S. are teaming up to tackle diabetic retinopathy, and the collaboration is looking pretty first class.
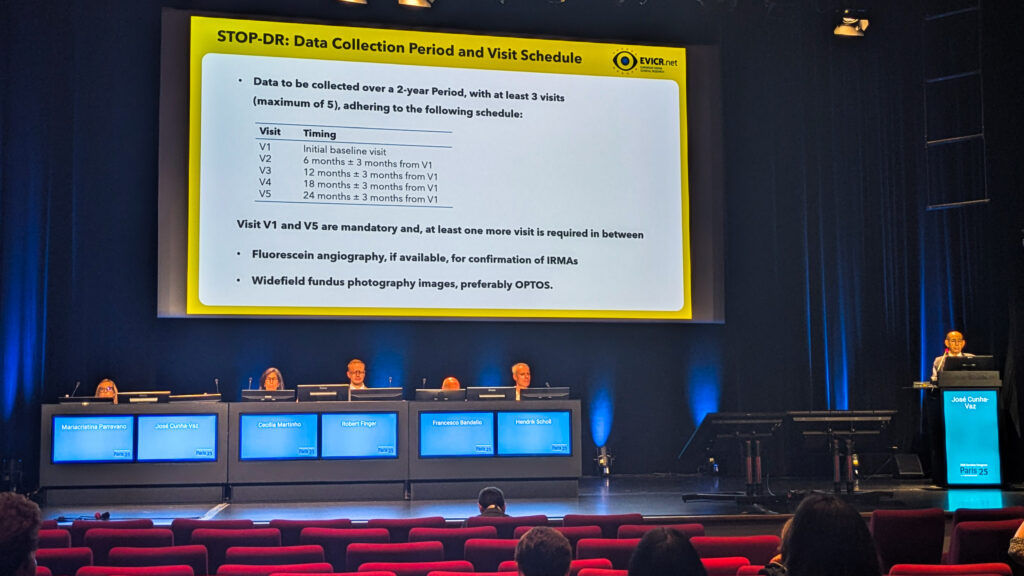
Prof. José Cunha-Vaz (Portugal) unveiled a new transatlantic partnership between EVICR.net and U.S.-based Diabetic Retinopathy Clinical Research (DRCR) Network.
“Everyone that works with diabetic retinopathy and is interested in clinical research…realizes the relevance of contributions of the DRCR network,” he said, tipping his hat to their track record.
READ MORE: The Promise of Gene Therapy in Retinal Disorders
Their maiden voyage together is Protocol AR, a natural history study zeroing in on retinal function in diabetic patients. And there’s also STOP ER, an imaging study with a sharp focus on intravitreal vascular abnormalities as early predictors of vision-threatening complications.
“The concept is to image transition from hypoperfusion to hyperperfusion, the response that leads to vision-threatening complications,” Prof. Cunha-Vaz explained. OCT-A will be the passport, with Boehringer Ingelheim (Germany) footing the travel bill.
Intermediate AMD hotspots
Intermediate age-related macular degeneration (AMD) doesn’t make headlines like its late-stage counterpart, but this is where the detective work gets interesting.
Prof. Sobha Sivaprasad (UK) showed up with new results from the INTERCEPT AMD study, a natural history investigation run across 26 centers in eight European countries, with backing from Boehringer Ingelheim.
Patients were sorted into four imaging categories, depending on whether incomplete retinal pigment epithelium and outer retinal atrophy (iRORA) or subretinal drusenoid deposits (SDD) appeared.

“Intermediate AMD patients have good vision,” Dr. Sivaprasad stressed. “If they do not have good vision, then you have to investigate as to why. They may not be related to intermediate AMD.”
Over two years, approximately 10% of eyes progressed to late AMD. The detective’s shortlist of suspects? Age over 75, presence of iRORA and fellow-eye neovascular AMD. Even a five-letter drop in visual acuity added an 11% increased risk of progression.
READ MORE: EURETINA 2025: What’s Next in the Neovascular AMD Playbook
Intermediate AMD endpoints on the horizon
Clinical trials can’t last forever, but without the right endpoints, they may as well.
Prof. Robert Finger (Germany) stepped up with news from MACUSTAR, the ambitious project on a mission to solve one of intermediate AMD’s biggest headaches: trial design.
“We need to find treatments, and for this we need to enable clinical trials,” he explained. “These clinical trials need suitable endpoints so that clinical trials will not run for 10 years with thousands of people to find the few who will respond and/or progress to late AMD.”
To that end, the study enrolled about 700 patients (most with intermediate AMD) and put them through an exhaustive test drive of structural endpoints, functional endpoints and even patient-reported outcomes. The goal? To lock down a reliable set of markers that regulators and researchers alike can actually work with.

And they aren’t leaving regulators guessing. “We have regular interaction. Three so far with EMA [European Medicines Agency] and one also with the FDA [U.S. Food and Drug Administration] and NICE [National Institute for Health and Care Excellence],” Prof. Finger reported.
That ongoing dialogue should help ensure whatever endpoints emerge are not only scientifically solid but also stamped with approval for future trial use.
READ MORE: The Slow Burn of Dry AMD at EURETINA 2025
Retinal dystrophy news
As if inherited retinal diseases weren’t complicated enough, there’s a brand new one in town.
Dr. Hendrik Scholl (Germany) presented news that the retinal dystrophy family just got a new member: lysosomal macular dystrophy. “This is a completely new entity that was just recently discovered… caused by loss of function mutations in three genes that all are subunits of the so-called vesicular AP5 complex,” he explained.
Clinically, it runs the gamut, from subtle flat maculopathy to severe chorioretinal atrophy, with onset sneaking in later than most would expect (around 45 on average). “Clearly, it’s an important differential diagnosis for macular disease,” Dr. Scholl added, making the case for keeping this newcomer on the diagnostic radar.
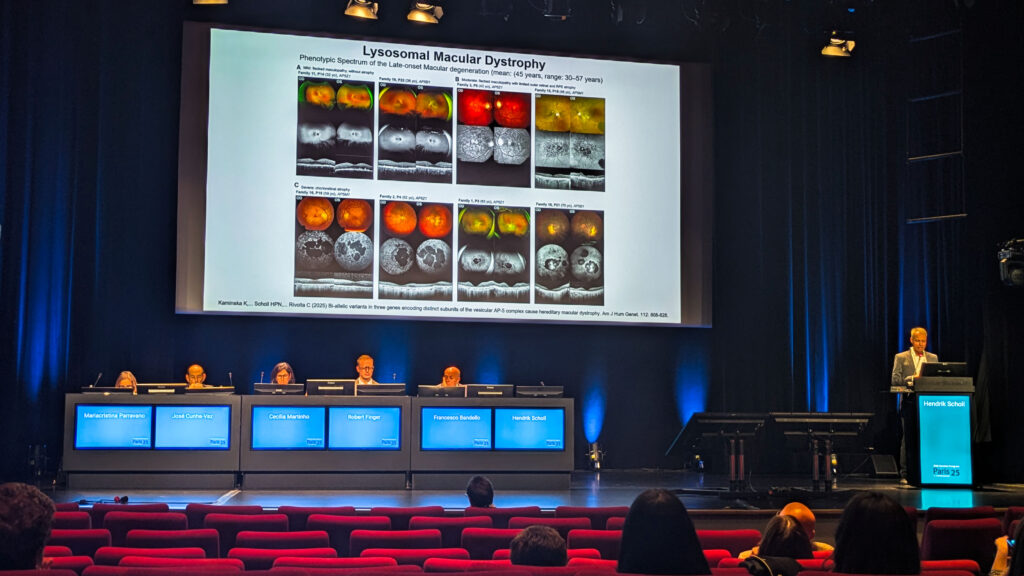
On the therapy front, he acknowledged that gene therapy for inherited retinal diseases (IRDs) has had its share of letdowns (ahem, bota-vec)…but not without reasons for optimism.
The DRAGON trial of oral tinlarebant (Belite Bio; California, USA) in adolescent Stargardt disease has stirred excitement. “The FDA provided breakthrough therapy designation for tinlarebant in the development of the first therapy ever for Stargardt disease,” he reported.
READ MORE: FDA Grants Breakthrough Therapy Designation to Belite Bio’s Tinlarebant for Stargardt Disease
PCV in Europe
Polypoidal choroidal vasculopathy (PCV) in Caucasian patients has long been a blind spot. Now, Europe is finally adjusting the focus.
Prof. Francesco Bandello (Italy) presented MONDEGO, a Phase IV EVICR.net study testing faricimab (Roche; Basel, Switzerland) in this under-studied population.
“Unfortunately, at the moment, we do not have a lot of information about this kind of disease in Caucasian patients,” he acknowledged.
The trial plans to recruit 120 patients across Latvia, Portugal, Spain and Italy, with best-corrected visual acuity change at 40 to 48 weeks as the primary endpoint.

Get the latest stories from the EURETINA 2025 here.
Editor’s Note: The 25th EURETINA Congress was held from 4-7 September, in Paris, France. Reporting for this story took place during the event. This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.